Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 October 2022

Health effects associated with smoking: a Burden of Proof study
- Xiaochen Dai ORCID: orcid.org/0000-0002-0289-7814 1 , 2 ,
- Gabriela F. Gil 1 ,
- Marissa B. Reitsma 1 ,
- Noah S. Ahmad 1 ,
- Jason A. Anderson 1 ,
- Catherine Bisignano 1 ,
- Sinclair Carr 1 ,
- Rachel Feldman 1 ,
- Simon I. Hay ORCID: orcid.org/0000-0002-0611-7272 1 , 2 ,
- Jiawei He 1 , 2 ,
- Vincent Iannucci 1 ,
- Hilary R. Lawlor 1 ,
- Matthew J. Malloy 1 ,
- Laurie B. Marczak 1 ,
- Susan A. McLaughlin 1 ,
- Larissa Morikawa ORCID: orcid.org/0000-0001-9749-8033 1 ,
- Erin C. Mullany 1 ,
- Sneha I. Nicholson 1 ,
- Erin M. O’Connell 1 ,
- Chukwuma Okereke 1 ,
- Reed J. D. Sorensen 1 ,
- Joanna Whisnant 1 ,
- Aleksandr Y. Aravkin 1 , 3 ,
- Peng Zheng 1 , 2 ,
- Christopher J. L. Murray ORCID: orcid.org/0000-0002-4930-9450 1 , 2 &
- Emmanuela Gakidou ORCID: orcid.org/0000-0002-8992-591X 1 , 2
Nature Medicine volume 28 , pages 2045–2055 ( 2022 ) Cite this article
31k Accesses
64 Citations
168 Altmetric
Metrics details
- Risk factors
Matters Arising to this article was published on 14 April 2023
As a leading behavioral risk factor for numerous health outcomes, smoking is a major ongoing public health challenge. Although evidence on the health effects of smoking has been widely reported, few attempts have evaluated the dose–response relationship between smoking and a diverse range of health outcomes systematically and comprehensively. In the present study, we re-estimated the dose–response relationships between current smoking and 36 health outcomes by conducting systematic reviews up to 31 May 2022, employing a meta-analytic method that incorporates between-study heterogeneity into estimates of uncertainty. Among the 36 selected outcomes, 8 had strong-to-very-strong evidence of an association with smoking, 21 had weak-to-moderate evidence of association and 7 had no evidence of association. By overcoming many of the limitations of traditional meta-analyses, our approach provides comprehensive, up-to-date and easy-to-use estimates of the evidence on the health effects of smoking. These estimates provide important information for tobacco control advocates, policy makers, researchers, physicians, smokers and the public.
Similar content being viewed by others
A systematic review and network meta-analysis of population-level interventions to tackle smoking behaviour
The Burden of Proof studies: assessing the evidence of risk
Health effects associated with exposure to secondhand smoke: a Burden of Proof study
Among both the public and the health experts, smoking is recognized as a major behavioral risk factor with a leading attributable health burden worldwide. The health risks of smoking were clearly outlined in a canonical study of disease rates (including lung cancer) and smoking habits in British doctors in 1950 and have been further elaborated in detail over the following seven decades 1 , 2 . In 2005, evidence of the health consequences of smoking galvanized the adoption of the first World Health Organization (WHO) treaty, the Framework Convention on Tobacco Control, in an attempt to drive reductions in global tobacco use and second-hand smoke exposure 3 . However, as of 2020, an estimated 1.18 billion individuals globally were current smokers and 7 million deaths and 177 million disability-adjusted life-years were attributed to smoking, reflecting a persistent public health challenge 4 . Quantifying the relationship between smoking and various important health outcomes—in particular, highlighting any significant dose–response relationships—is crucial to understanding the attributable health risk experienced by these individuals and informing responsive public policy.
Existing literature on the relationship between smoking and specific health outcomes is prolific, including meta-analyses, cohort studies and case–control studies analyzing the risk of outcomes such as lung cancer 5 , 6 , 7 , chronic obstructive pulmonary disease (COPD) 8 , 9 , 10 and ischemic heart disease 11 , 12 , 13 , 14 due to smoking. There are few if any attempts, however, to systematically and comprehensively evaluate the landscape of evidence on smoking risk across a diverse range of health outcomes, with most current research focusing on risk or attributable burden of smoking for a specific condition 7 , 15 , thereby missing the opportunity to provide a comprehensive picture of the health risk experienced by smokers. Furthermore, although evidence surrounding specific health outcomes, such as lung cancer, has generated widespread consensus, findings about the attributable risk of other outcomes are much more heterogeneous and inconclusive 16 , 17 , 18 . These studies also vary in their risk definitions, with many comparing dichotomous exposure measures of ever smokers versus nonsmokers 19 , 20 . Others examine the distinct risks of current smokers and former smokers compared with never smokers 21 , 22 , 23 . Among the studies that do analyze dose–response relationships, there is large variation in the units and dose categories used in reporting their findings (for example, the use of pack-years or cigarettes per day) 24 , 25 , which complicates the comparability and consolidation of evidence. This, in turn, can obscure data that could inform personal health choices, public health practices and policy measures. Guidance on the health risks of smoking, such as the Surgeon General’s Reports on smoking 26 , 27 , is often based on experts’ evaluation of heterogenous evidence, which, although extremely useful and well suited to carefully consider nuances in the evidence, is fundamentally subjective.
The present study, as part of the Global Burden of Diseases, Risk Factors, and Injuries Study (GBD) 2020, re-estimated the continuous dose–response relationships (the mean risk functions and associated uncertainty estimates) between current smoking and 36 health outcomes (Supplementary Table 1 ) by identifying input studies using a systematic review approach and employing a meta-analytic method 28 . The 36 health outcomes that were selected based on existing evidence of a relationship included 16 cancers (lung cancer, esophageal cancer, stomach cancer, leukemia, liver cancer, laryngeal cancer, breast cancer, cervical cancer, colorectal cancer, lip and oral cavity cancer, nasopharyngeal cancer, other pharynx cancer (excluding nasopharynx cancer), pancreatic cancer, bladder cancer, kidney cancer and prostate cancer), 5 cardiovascular diseases (CVDs: ischemic heart disease, stroke, atrial fibrillation and flutter, aortic aneurysm and peripheral artery disease) and 15 other diseases (COPD, lower respiratory tract infections, tuberculosis, asthma, type 2 diabetes, Alzheimer’s disease and related dementias, Parkinson’s disease, multiple sclerosis, cataracts, gallbladder diseases, low back pain, peptic ulcer disease, rheumatoid arthritis, macular degeneration and fractures). Definitions of the outcomes are described in Supplementary Table 1 . We conducted a separate systematic review for each risk–outcome pair with the exception of cancers, which were done together in a single systematic review. This approach allowed us to systematically identify all relevant studies indexed in PubMed up to 31 May 2022, and we extracted relevant data on risk of smoking, including study characteristics, following a pre-specified template (Supplementary Table 2 ). The meta-analytic tool overcomes many of the limitations of traditional meta-analyses by incorporating between-study heterogeneity into the uncertainty of risk estimates, accounting for small numbers of studies, relaxing the assumption of log(linearity) applied to the risk functions, handling differences in exposure ranges between comparison groups, and systematically testing and adjusting for bias due to study designs and characteristics. We then estimated the burden-of-proof risk function (BPRF) for each risk–outcome pair, as proposed by Zheng et al. 29 ; the BPRF is a conservative risk function defined as the 5th quantile curve (for harmful risks) that reflects the smallest harmful effect at each level of exposure consistent with the available evidence. Given all available data for each outcome, the risk of smoking is at least as harmful as the BPRF indicates.
We used the BPRF for each risk–outcome pair to calculate risk–outcome scores (ROSs) and categorize the strength of evidence for the association between smoking and each health outcome using a star rating from 1 to 5. The interpretation of the star ratings is as follows: 1 star (*) indicates no evidence of association; 2 stars (**) correspond to a 0–15% increase in risk across average range of exposures for harmful risks; 3 stars (***) represent a 15–50% increase in risk; 4 stars (****) refer to >50–85% increase in risk; and 5 stars (*****) equal >85% increase in risk. The thresholds for each star rating were developed in consultation with collaborators and other stakeholders.
The increasing disease burden attributable to current smoking, particularly in low- and middle-income countries 4 , demonstrates the relevance of the present study, which quantifies the strength of the evidence using an objective, quantitative, comprehensive and comparative framework. Findings from the present study can be used to support policy makers in making informed smoking recommendations and regulations focusing on the associations for which the evidence is strongest (that is, the 4- and 5-star associations). However, associations with a lower star rating cannot be ignored, especially when the outcome has high prevalence or severity. A summary of the main findings, limitations and policy implications of the study is presented in Table 1 .
We evaluated the mean risk functions and the BPRFs for 36 health outcomes that are associated with current smoking 30 (Table 2 ). Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 31 for each of our systematic reviews, we identified studies reporting relative risk (RR) of incidence or mortality from each of the 36 selected outcomes for smokers compared with nonsmokers. We reviewed 21,108 records, which were identified to have been published between 1 May 2018 and 31 May 2022; this represents the most recent time period since the last systematic review of the available evidence for the GBD at the time of publication. The meta-analyses reported in the present study for each of the 36 health outcomes are based on evidence from a total of 793 studies published between 1970 and 2022 (Extended Data Fig. 1 – 5 and Supplementary Information 1.5 show the PRISMA diagrams for each outcome). Only prospective cohort and case–control studies were included for estimating dose–response risk curves, but cross-sectional studies were also included for estimating the age pattern of smoking risk on cardiovascular and circulatory disease (CVD) outcomes. Details on each, including the study’s design, data sources, number of participants, length of follow-up, confounders adjusted for in the input data and bias covariates included in the dose–response risk model, can be found in Supplementary Information 2 and 3 . The theoretical minimum risk exposure level used for current smoking was never smoking or zero 30 .
Five-star associations
When the most conservative interpretation of the evidence, that is, the BPRF, suggests that the average exposure (15th–85th percentiles of exposure) of smoking increases the risk of a health outcome by >85% (that is, ROS > 0.62), smoking and that outcome are categorized as a 5-star pair. Among the 36 outcomes, there are 5 that have a 5-star association with current smoking: laryngeal cancer (375% increase in risk based on the BPRF, 1.56 ROS), aortic aneurysm (150%, 0.92), peripheral artery disease (137%, 0.86), lung cancer (107%, 0.73) and other pharynx cancer (excluding nasopharynx cancer) (92%, 0.65).
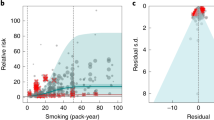
Results for all 5-star risk–outcome pairs are available in Table 2 and Supplementary Information 4.1 . In the present study, we provide detailed results for one example 5-star association: current smoking and lung cancer. We extracted 371 observations from 25 prospective cohort studies and 53 case–control studies across 25 locations (Supplementary Table 3 ) 5 , 6 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 . Exposure ranged from 1 pack-year to >112 pack-years, with the 85th percentile of exposure being 50.88 pack-years (Fig. 1a ).
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on the x axis and the estimated s.d. that includes reported s.d. and between-study heterogeneity on the y axis.
We found a very strong and significant harmful relationship between pack-years of current smoking and the RR of lung cancer (Fig. 1b ). The mean RR of lung cancer at 20 pack-years of smoking was 5.11 (95% uncertainty interval (UI) inclusive of between-study heterogeneity = 1.84–14.99). At 50.88 pack-years (85th percentile of exposure), the mean RR of lung cancer was 13.42 (2.63–74.59). See Table 2 for mean RRs at other exposure levels. The BPRF, which represents the most conservative interpretation of the evidence (Fig. 1a ), suggests that smoking in the 15th–85th percentiles of exposure increases the risk of lung cancer by an average of 107%, yielding an ROS of 0.73.
The relationship between pack-years of current smoking and RR of lung cancer is nonlinear, with diminishing impact of further pack-years of smoking, particularly for middle-to-high exposure levels (Fig. 1b ). To reduce the effect of bias, we adjusted observations that did not account for more than five confounders, including age and sex, because they were the significant bias covariates identified by the bias covariate selection algorithm 29 (Supplementary Table 7 ). The reported RRs across studies were very heterogeneous. Our meta-analytic method, which accounts for the reported uncertainty in both the data and between-study heterogeneity, fit the data and covered the estimated residuals well (Fig. 1c ). After trimming 10% of outliers, we still detected publication bias in the results for lung cancer. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 5-star pairs.
Four-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of a health outcome by 50–85% (that is, ROS > 0.41–0.62), smoking is categorized as having a 4-star association with that outcome. We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer (52%, 0.42).
In the present study, we provide detailed results for one example 4-star association: current smoking and COPD. We extracted 51 observations from 11 prospective cohort studies and 4 case–control studies across 36 locations (Supplementary Table 3 ) 6 , 8 , 9 , 10 , 78 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 . Exposure ranged from 1 pack-year to 100 pack-years, with the 85th percentile of exposure in the exposed group being 49.75 pack-years.
We found a strong and significant harmful relationship between pack-years of current smoking and RR of COPD (Fig. 2b ). The mean RR of COPD at 20 pack-years was 3.17 (1.60–6.55; Table 2 reports RRs at other exposure levels). At the 85th percentile of exposure, the mean RR of COPD was 6.01 (2.08–18.58). The BPRF suggests that average smoking exposure raises the risk of COPD by an average of 72%, yielding an ROS of 0.54. The results for the other health outcomes that have an association with smoking rated as 4 stars are shown in Table 2 and Supplementary Information 4.2 .
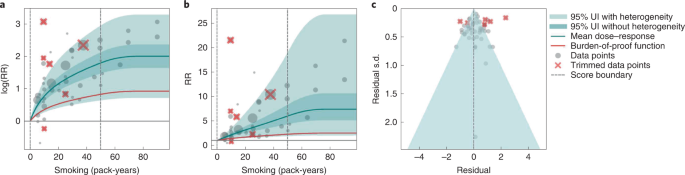
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on th e x axis and the estimated s.d. that includes the reported s.d. and between-study heterogeneity on the y axis.
The relationship between smoking and COPD is nonlinear, with diminishing impact of further pack-years of current smoking on risk of COPD, particularly for middle-to-high exposure levels (Fig. 2a ). To reduce the effect of bias, we adjusted observations that did not account for age and sex and/or were generated for individuals aged >65 years 116 , because they were the two significant bias covariates identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was large heterogeneity in the reported RRs across studies, and our meta-analytic method fit the data and covered the estimated residuals well (Fig. 2b ). Although we trimmed 10% of outliers, publication bias was still detected in the results for COPD. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for reported RR data and alternative exposures across studies for the remaining health outcomes that have a 4-star association with smoking.
Three-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of a health outcome by 15–50% (or, when protective, decreases the risk of an outcome by 13–34%; that is, ROS >0.14–0.41), the association between smoking and that outcome is categorized as having a 3-star rating. We identified 15 outcomes with a 3-star association: bladder cancer (40% increase in risk, 0.34 ROS); tuberculosis (31%, 0.27); esophageal cancer (29%, 0.26); cervical cancer, multiple sclerosis and rheumatoid arthritis (each 23–24%, 0.21); lower back pain (22%, 0.20); ischemic heart disease (20%, 0.19); peptic ulcer and macular degeneration (each 19–20%, 0.18); Parkinson's disease (protective risk, 15% decrease in risk, 0.16); and stomach cancer, stroke, type 2 diabetes and cataracts (each 15–17%, 0.14–0.16).
We present the findings on smoking and type 2 diabetes as an example of a 3-star risk association. We extracted 102 observations from 24 prospective cohort studies and 4 case–control studies across 15 locations (Supplementary Table 3 ) 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 . The exposure ranged from 1 cigarette to 60 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 26.25 cigarettes smoked per day.
We found a moderate and significant harmful relationship between cigarettes smoked per day and the RR of type 2 diabetes (Fig. 3b ). The mean RR of type 2 diabetes at 20 cigarettes smoked per day was 1.49 (1.18–1.90; see Table 2 for other exposure levels). At the 85th percentile of exposure, the mean RR of type 2 diabetes was 1.54 (1.20–2.01). The BPRF suggests that average smoking exposure raises the risk of type 2 diabetes by an average of 16%, yielding an ROS of 0.15. See Table 2 and Supplementary Information 4.3 for results for the additional health outcomes with an association with smoking rated as 3 stars.
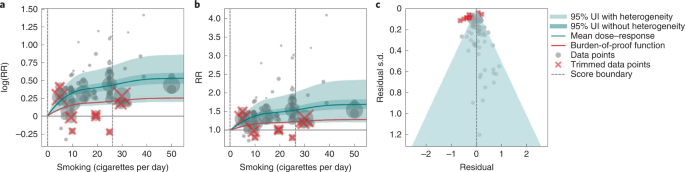
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on the x axis and the estimated s.d. that includes the reported s.d. and between-study heterogeneity on the y axis.
The relationship between smoking and type 2 diabetes is nonlinear, particularly for high exposure levels where the mean risk curve becomes flat (Fig. 3a ). We adjusted observations that were generated in subpopulations, because it was the only significant bias covariate identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was moderate heterogeneity in the observed RR data across studies and our meta-analytic method fit the data and covered the estimated residuals extremely well (Fig. 3b,c ). After trimming 10% of outliers, we still detected publication bias in the results for type 2 diabetes. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 3-star pairs.
Two-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of an outcome by 0–15% (that is, ROS 0.0–0.14), the association between smoking and that outcome is categorized as a 2-star rating. We identified six 2-star outcomes: nasopharyngeal cancer (14% increase in risk, 0.13 ROS); Alzheimer’s and other dementia (10%, 0.09); gallbladder diseases and atrial fibrillation and flutter (each 6%, 0.06); lip and oral cavity cancer (5%, 0.05); and breast cancer (4%, 0.04).
We present the findings on smoking and breast cancer as an example of a 2-star association. We extracted 93 observations from 14 prospective cohort studies and 9 case–control studies across 14 locations (Supplementary Table 3 ) 84 , 87 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 . The exposure ranged from 1 cigarette to >76 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 34.10 cigarettes smoked per day.
We found a weak but significant relationship between pack-years of current smoking and RR of breast cancer (Extended Data Fig. 6 ). The mean RR of breast cancer at 20 pack-years was 1.17 (1.04–1.31; Table 2 reports other exposure levels). The BPRF suggests that average smoking exposure raises the risk of breast cancer by an average of 4%, yielding an ROS of 0.04. See Table 2 and Supplementary Information 4.4 for results on the additional health outcomes for which the association with smoking has been categorized as 2 stars.
The relationship between smoking and breast cancer is nonlinear, particularly for high exposure levels where the mean risk curve becomes flat (Extended Data Fig. 6a ). To reduce the effect of bias, we adjusted observations that were generated in subpopulations, because it was the only significant bias covariate identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was heterogeneity in the reported RRs across studies, but our meta-analytic method fit the data and covered the estimated residuals (Extended Data Fig. 6b ). After trimming 10% of outliers, we did not detect publication bias in the results for breast cancer. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 2-star pairs.
One-star associations
When average exposure to smoking does not significantly increase (or decrease) the risk of an outcome, once between-study heterogeneity and other sources of uncertainty are accounted for (that is, ROS < 0), the association between smoking and that outcome is categorized as 1 star, indicating that there is not sufficient evidence for the effect of smoking on the outcome to reject the null (that is, there may be no association). There were seven outcomes with an association with smoking that rated as 1 star: colorectal and kidney cancer (each –0.01 ROS); leukemia (−0.04); fractures (−0.05); prostate cancer (−0.06); liver cancer (−0.32); and asthma (−0.64).
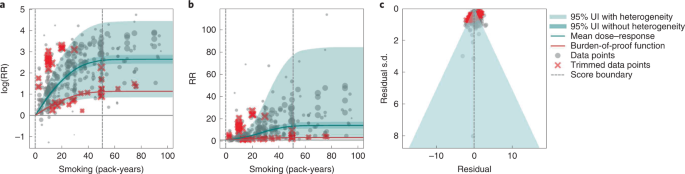
We use smoking and prostate cancer as examples of a 1-star association. We extracted 78 observations from 21 prospective cohort studies and 1 nested case–control study across 15 locations (Supplementary Table 3 ) 157 , 160 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 . The exposure among the exposed group ranged from 1 cigarette to 90 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 29.73 cigarettes smoked per day.
Based on our conservative interpretation of the data, we did not find a significant relationship between cigarettes smoked per day and the RR of prostate cancer (Fig. 4B ). The exposure-averaged BPRF for prostate cancer was 0.94, which was opposite null from the full range of mean RRs, such as 1.16 (0.89–1.53) at 20 cigarettes smoked per day. The corresponding ROS was −0.06, which is consistent with no evidence of an association between smoking and increased risk of prostate cancer. See Table 2 and Supplementary Information 4.5 for results for the additional outcomes that have a 1-star association with smoking.
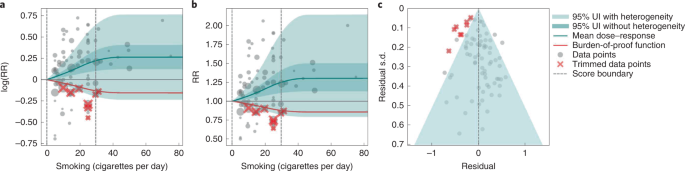
The relationship between smoking and prostate cancer is nonlinear, particularly for middle-to-high exposure levels where the mean risk curve becomes flat (Fig. 4a ). We did not adjust for any bias covariate because no significant bias covariates were selected by the algorithm (Supplementary Table 7 ). The RRs reported across studies were very heterogeneous, but our meta-analytic method fit the data and covered the estimated residuals well (Fig. 4b,c ). The ROS associated with the BPRF is −0.05, suggesting that the most conservative interpretation of all evidence, after accounting for between-study heterogeneity, indicates an inconclusive relationship between smoking exposure and the risk of prostate cancer. After trimming 10% of outliers, we still detected publication bias in the results for prostate cancer, which warrants further studies using sample populations. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 1-star pairs.
Age-specific dose–response risk for CVD outcomes
We produced age-specific dose–response risk curves for the five selected CVD outcomes ( Methods ). The ROS associated with each smoking–CVD pair was calculated based on the reference risk curve estimated using all risk data regardless of age information. Estimation of the BPRF, calculation of the associated ROS and star rating of the smoking–CVD pairs follow the same rules as the other non-CVD smoking–outcome pairs (Table 1 and Supplementary Figs. 2 – 4 ). Once we had estimated the reference dose–response risk curve for each CVD outcome, we determined the age group of the reference risk curve. The reference age group is 55–59 years for all CVD outcomes, except for peripheral artery disease, the reference age group for which is 60–64 years. We then estimated the age pattern of smoking on all CVD outcomes (Supplementary Fig. 2 ) and calculated age attenuation factors of the risk for each age group by comparing the risk of each age group with that of the reference age group, using the estimated age pattern (Supplementary Fig. 3 ). Last, we applied the draws of age attenuation factors of each age group to the dose–response risk curve for the reference age group to produce the age group-specific dose–response risk curves for each CVD outcome (Supplementary Fig. 4 ).
Using our burden-of-proof meta-analytic methods, we re-estimated the dose–response risk of smoking on 36 health outcomes that had previously been demonstrated to be associated with smoking 30 , 186 . Using these methods, which account for both the reported uncertainty of the data and the between-study heterogeneity, we found that 29 of the 36 smoking–outcome pairs are supported by evidence that suggests a significant dose–response relationship between smoking and the given outcome (28 with a harmful association and 1 with a protective association). Conversely, after accounting for between-study heterogeneity, the available evidence of smoking risk on seven outcomes (that is, colon and rectum cancer, kidney cancer, leukemia, prostate cancer, fractures, liver cancer and asthma) was insufficient to reject the null or draw definitive conclusions on their relationship to smoking. Among the 29 outcomes that have evidence supporting a significant relationship to smoking, 8 had strong-to-very-strong evidence of a relationship, meaning that, given all the available data on smoking risk, we estimate that average exposure to smoking increases the risk of those outcomes by >50% (4- and 5-star outcomes). The currently available evidence for the remaining 21 outcomes with a significant association with current smoking was weak to moderate, indicating that smoking increases the risk of those outcomes by at least >0–50% (2- and 3-star associations).
Even under our conservative interpretation of the data, smoking is irrefutably harmful to human health, with the greatest increases in risk occurring for laryngeal cancer, aortic aneurysm, peripheral artery disease, lung cancer and other pharynx cancer (excluding nasopharynx cancer), which collectively represent large causes of death and ill-health. The magnitude of and evidence for the associations between smoking and its leading health outcomes are among the highest currently analyzed in the burden-of-proof framework 29 . The star ratings assigned to each smoking–outcome pair offer policy makers a way of categorizing and comparing the evidence for a relationship between smoking and its potential health outcomes ( https://vizhub.healthdata.org/burden-of-proof ). We found that, for seven outcomes in our analysis, there was insufficient or inconsistent evidence to demonstrate a significant association with smoking. This is a key finding because it demonstrates the need for more high-quality data for these particular outcomes; availability of more data should improve the strength of evidence for whether or not there is an association between smoking and these health outcomes.
Our systematic review approach and meta-analytic methods have numerous benefits over existing systematic reviews and meta-analyses on the same topic that use traditional random effects models. First, our approach relaxes the log(linear) assumption, using a spline ensemble to estimate the risk 29 . Second, our approach allows variable reference groups and exposure ranges, allowing for more accurate estimates regardless of whether or not the underlying relative risk is log(linear). Furthermore, it can detect outliers in the data automatically. Finally, it quantifies uncertainty due to between-study heterogeneity while accounting for small numbers of studies, minimizing the risk that conclusions will be drawn based on spurious findings.
We believe that the results for the association between smoking and each of the 36 health outcomes generated by the present study, including the mean risk function, BPRF, ROS, average excess risk and star rating, could be useful to a range of stakeholders. Policy makers can formulate their decisions on smoking control priorities and resource allocation based on the magnitude of the effect and the consistency of the evidence relating smoking to each of the 36 outcomes, as represented by the ROS and star rating for each smoking–outcome association 187 . Physicians and public health practitioners can use the estimates of average increased risk and the star rating to educate patients and the general public about the risk of smoking and to promote smoking cessation 188 . Researchers can use the estimated mean risk function or BPRF to obtain the risk of an outcome at a given smoking exposure level, as well as uncertainty surrounding that estimate of risk. The results can also be used in the estimation of risk-attributable burden, that is, the deaths and disability-adjusted life-years due to each outcome that are attributable to smoking 30 , 186 . For the general public, these results could help them to better understand the risk of smoking and manage their health 189 .
Although our meta-analysis was comprehensive and carefully conducted, there are limitations to acknowledge. First, the bias covariates used, although carefully extracted and evaluated, were based on observable study characteristics and thus may not fully capture unobserved characteristics such as study quality or context, which might be major sources of bias. Second, if multiple risk estimates with different adjustment levels were reported in a given study, we included only the fully adjusted risk estimate and modeled the adjustment level according to the number of covariates adjusted for (rather than which covariates were adjusted for) and whether a standard adjustment for age and sex had been applied. This approach limited our ability to make full use of all available risk estimates in the literature. Third, although we evaluated the potential for publication bias in the data, we did not test for other forms of bias such as when studies are more consistent with each other than expected by chance 29 . Fourth, our analysis assumes that the relationships between smoking and health outcomes are similar across geographical regions and over time. We do not have sufficient evidence to quantify how the relationships may have evolved over time because the composition of smoking products has also changed over time. Perhaps some of the heterogeneity of the effect sizes in published studies reflects this; however, this cannot be discerned with the currently available information.
In the future, we plan to include crude and partially adjusted risk estimates in our analyses to fully incorporate all available risk estimates, to model the adjusted covariates in a more comprehensive way by mapping the adjusted covariates across all studies comprehensively and systematically, and to develop methods to evaluate additional forms of potential bias. We plan to update our results on a regular basis to provide timely and up-to-date evidence to stakeholders.
To conclude, we have re-estimated the dose–response risk of smoking on 36 health outcomes while synthesizing all the available evidence up to 31 May 2022. We found that, even after factoring in the heterogeneity between studies and other sources of uncertainty, smoking has a strong-to-very-strong association with a range of health outcomes and confirmed that smoking is irrefutably highly harmful to human health. We found that, due to small numbers of studies, inconsistency in the data, small effect sizes or a combination of these reasons, seven outcomes for which some previous research had found an association with smoking did not—under our meta-analytic framework and conservative approach to interpreting the data—have evidence of an association. Our estimates of the evidence for risk of smoking on 36 selected health outcomes have the potential to inform the many stakeholders of smoking control, including policy makers, researchers, public health professionals, physicians, smokers and the general public.
For the present study, we used a meta-analytic tool, MR-BRT (metaregression—Bayesian, regularized, trimmed), to estimate the dose–response risk curves of the risk of a health outcome across the range of current smoking levels along with uncertainty estimates 28 . Compared with traditional meta-analysis using linear mixed effect models, MR-BRT relaxes the assumption of a log(linear) relationship between exposure and risk, incorporates between-study heterogeneity into the uncertainty of risk estimates, handles estimates reported across different exposure categories, automatically identifies and trims outliers, and systematically tests and adjusts for bias due to study designs and characteristics. The meta-analytic methods employed by the present study followed the six main steps proposed by Zheng et al. 28 , 29 , namely: (1) enacting a systematic review approach and data extraction following a pre-specified and standardized protocol; (2) estimating the shape of the relationship between exposure and RR; (3) evaluating and adjusting for systematic bias as a function of study characteristics and risk estimation; (4) quantifying between-study heterogeneity while adjusting for within-study correlation and the number of studies; (5) evaluating potential publication or reporting biases; and (6) estimating the mean risk function and the BPRF, calculating the ROS and categorizing smoking–outcome pairs using a star-rating scheme from 1 to 5.
The estimates for our primary indicators of this work—mean RRs across a range of exposures, BRPFs, ROSs and star ratings for each risk–outcome pair—are not specific to or disaggregated by specific populations. We did not estimate RRs separately for different locations, sexes (although the RR of prostate cancer was estimated only for males and of cervical and breast cancer only for females) or age groups (although this analysis was applied to disease endpoints in adults aged ≥30 years only and, as detailed below, age-specific estimates were produced for the five CVD outcomes).
The present study complies with the PRISMA guidelines 190 (Supplementary Tables 9 and 10 and Supplementary Information 1.5 ) and Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations 191 (Supplementary Table 11 ). The study was approved by the University of Washington Institutional Review Board (study no. 9060). The systematic review approach was not registered.
Selecting health outcomes
In the present study, current smoking is defined as the current use of any smoked tobacco product on a daily or occasional basis. Health outcomes were initially selected using the World Cancer Research Fund criteria for convincing or probable evidence as described in Murray et al. 186 . The 36 health outcomes that were selected based on existing evidence of a relationship included 16 cancers (lung cancer, esophageal cancer, stomach cancer, leukemia, liver cancer, laryngeal cancer, breast cancer, cervical cancer, colorectal cancer, lip and oral cavity cancer, nasopharyngeal cancer, other pharynx cancer (excluding nasopharynx cancer), pancreatic cancer, bladder cancer, kidney cancer and prostate cancer), 5 CVDs (ischemic heart disease, stroke, atrial fibrillation and flutter, aortic aneurysm and peripheral artery disease) and 15 other diseases (COPD, lower respiratory tract infections, tuberculosis, asthma, type 2 diabetes, Alzheimer’s disease and related dementias, Parkinson’s disease, multiple sclerosis, cataracts, gallbladder diseases, low back pain, peptic ulcer disease, rheumatoid arthritis, macular degeneration and fracture). Definitions of the outcomes are described in Supplementary Table 1 .
Step 1: systematic review approach to literature search and data extraction
Informed by the systematic review approach we took for the GBD 2019 (ref. 30 ), for the present study we identified input studies in the literature using a systematic review approach for all 36 smoking–outcome pairs using updated search strings to identify all relevant studies indexed in PubMed up to 31 May 2022 and extracted data on smoking risk estimates. Briefly, the studies that were extracted represented several types of study design (for example, cohort and case–control studies), measured exposure in several different ways and varied in their choice of reference categories (where some compared current smokers with never smokers, whereas others compared current smokers with nonsmokers or former smokers). All these study characteristics were catalogued systematically and taken into consideration during the modeling part of the analysis.
In addition, for CVD outcomes, we also estimated the age pattern of risk associated with smoking. We applied a systematic review of literature approach for smoking risk for the five CVD outcomes. We developed a search string to search for studies reporting any association between binary smoking status (that is, current, former and ever smokers) and the five CVD outcomes from 1 January 1970 to 31 May 2022, and included only studies reporting age-specific risk (RR, odds ratio (OR), hazard ratio (HR)) of smoking status. The inclusion criteria and results of the systematic review approach are reported in accordance with PRISMA guidelines 31 . Details for each outcome on the search string used in the systematic review approach, refined inclusion and exclusion criteria, data extraction template and PRISMA diagram are given in Supplementary Information 1 . Title and/or abstract screening, full text screening and data extraction were conducted by 14 members of the research team and extracted data underwent manual quality assurance by the research team to verify accuracy.
Selecting exposure categories
Cumulative exposure in pack-years was the measure of exposure used for COPD and all cancer outcomes except for prostate cancer, to reflect the risk of both duration and intensity of current smoking on these outcomes. For prostate cancer, CVDs and all the other outcomes except for fractures, we used cigarette-equivalents smoked per day as the exposure for current smoking, because smoking intensity is generally thought to be more important than duration for these outcomes. For fractures, we used binary exposure, because there were few studies examining intensity or duration of smoking on fractures. The smoking–outcome pairs and the corresponding exposures are summarized in Supplementary Table 4 and are congruent with the GBD 2019 (refs. 30 , 186 ).
Steps 2–5: modeling dose–response RR of smoking on the selected health outcomes
Of the six steps proposed by Zheng et al. 29 , steps 2–5 cover the process of modeling dose–response risk curves. In step 2, we estimated the shape (or the ‘signal’) of the dose–response risk curves, integrating over different exposure ranges. To relax the log(linear) assumption usually applied to continuous dose–response risk and make the estimates robust to the placement of spline knots, we used an ensemble spline approach to fit the functional form of the dose–response relationship. The final ensemble model was a weighted combination of 50 models with random knot placement, with the weight of each model proportional to measures of model fit and total variation. To avoid the influence of extreme data and reduce publication bias, we trimmed 10% of data for each outcome as outliers. We also applied a monotonicity constraint to ensure that the mean risk curves were nondecreasing (or nonincreasing in the case of Parkinson’s disease).
In step 3, following the GRADE approach 192 , 193 , we quantified risk of bias across six domains, namely, representativeness of the study population, exposure, outcome, reverse causation, control for confounding and selection bias. Details about the bias covariates are provided in Supplementary Table 4 . We systematically tested for the effect of bias covariates using metaregression, selected significant bias covariates using the Lasso approach 194 , 195 and adjusted for the selected bias covariates in the final risk curve.
In step 4, we quantified between-study heterogeneity accounting for within-study correlation, uncertainty of the heterogeneity, as well as small number of studies. Specifically, we used a random intercept in the mixed-effects model to account for the within-study correlation and used a study-specific random slope with respect to the ‘signal’ to capture between-study heterogeneity. As between-study heterogeneity can be underestimated or even zero when the number of studies is small 196 , 197 , we used Fisher’s information matrix to estimate the uncertainty of the heterogeneity 198 and incorporated that uncertainty into the final results.
In step 5, in addition to generating funnel plots and visually inspecting for asymmetry (Figs. 1c , 2c , 3c and 4c and Extended Data Fig. 6c ) to identify potential publication bias, we also statistically tested for potential publication or reporting bias using Egger’s regression 199 . We flagged potential publication bias in the data but did not correct for it, which is in line with the general literature 10 , 200 , 201 . Full details about the modeling process have been published elsewhere 29 and model specifications for each outcome are in Supplementary Table 6 .
Step 6: estimating the mean risk function and the BPRF
In the final step, step 6, the metaregression model inclusive of the selected bias covariates from step 3 (for example, the highest adjustment level) was used to predict the mean risk function and its 95% UI, which incorporated the uncertainty of the mean effect, between-study heterogeneity and the uncertainty in the heterogeneity estimate accounting for small numbers of studies. Specifically, 1,000 draws were created for each 0.1 level of doses from 0 pack-years to 100 pack-years or cigarette-equivalents smoked per day using the Bayesian metaregression model. The mean of the 1,000 draws was used to estimate the mean risk at each exposure level, and the 25th and 95th draws were used to estimate the 95% UIs for the mean risk at each exposure level.
The BPRF 29 is a conservative estimate of risk function consistent with the available evidence, correcting for both between-study heterogeneity and systemic biases related to study characteristics. The BPRF is defined as either the 5th (if harmful) or 95th (if protective) quantile curve closest to the line of log(RR) of 0, which defines the null (Figs. 1a , 2b , 3a and 4a ). The BPRF represents the smallest harmful (or protective) effect of smoking on the corresponding outcome at each level of exposure that is consistent with the available evidence. A BPRF opposite null from the mean risk function indicates that insufficient evidence is available to reject null, that is, that there may not be an association between risk and outcome. Likewise, the further the BPRF is from null on the same side of null as the mean risk function, the higher the magnitude and evidence for the relationship. The BPRF can be interpreted as indicating that, even accounting for between-study heterogeneity and its uncertainty, the log(RR) across the studied smoking range is at least as high as the BPRF (or at least as low as the BPRF for a protective risk).
To quantify the strength of the evidence, we calculated the ROS for each smoking–outcome association as the signed value of the log(BPRF) averaged between the 15th and 85th percentiles of observed exposure levels for each outcome. The ROS is a single summary of the effect of smoking on the outcome, with higher positive ROSs corresponding to stronger and more consistent evidence and a higher average effect size of smoking and a negative ROS, suggesting that, based on the available evidence, there is no significant effect of smoking on the outcome after accounting for between-study heterogeneity.
For ease of communication, we further classified each smoking–outcome association into a star rating from 1 to 5. Briefly, 1-star associations have an ROS <0, indicating that there is insufficient evidence to find a significant association between smoking and the selected outcome. We divided the positive ROSs into ranges 0.0–0.14 (2-star), >0.14–0.41 (3-star), >0.41–0.62 (4-star) and >0.62 (5-star). These categories correspond to excess risk ranges for harmful risks of 0–15%, >15–50%, >50–85% and >85%. For protective risks, the ranges of exposure-averaged decreases in risk by star rating are 0–13% (2 stars), >13–34% (3 stars), >34–46% (4 stars) and >46% (5 stars).
Among the 36 smoking–outcome pairs analyzed, smoking fracture was the only binary risk–outcome pair, which was due to limited data on the dose–response risk of smoking on fracture 202 . The estimation of binary risk was simplified because the RR was merely a comparison between current smokers and nonsmokers or never smokers. The concept of ROS for continuous risk can naturally extend to binary risk because the BPRF is still defined as the 5th percentile of the effect size accounting for data uncertainty and between-study heterogeneity. However, binary ROSs must be divided by 2 to make them comparable with continuous ROSs, which were calculated by averaging the risk over the range between the 15th and the 85th percentiles of observed exposure levels. Full details about estimating mean risk functions, BPRFs and ROSs for both continuous and binary risk–outcome pairs can be found elsewhere 29 .
Estimating the age-specific risk function for CVD outcomes
For non-CVD outcomes, we assumed that the risk function was the same for all ages and all sexes, except for breast, cervical and prostate cancer, which were assumed to apply only to females or males, respectively. As the risk of smoking on CVD outcomes is known to attenuate with increasing age 203 , 204 , 205 , 206 , we adopted a four-step approach for GBD 2020 to produce age-specific dose–response risk curves for CVD outcomes.
First, we estimated the reference dose–response risk of smoking for each CVD outcome using dose-specific RR data for each outcome regardless of the age group information. This step was identical to that implemented for the other non-CVD outcomes. Once we had generated the reference curve, we determined the age group associated with it by calculating the weighted mean age across all dose-specific RR data (weighted by the reciprocal of the s.e.m. of each datum). For example, if the weighted mean age of all dose-specific RR data was 56.5, we estimated the age group associated with the reference risk curve to be aged 55–59 years. For cohort studies, the age range associated with the RR estimate was calculated as a mean age at baseline plus the mean/median years of follow-up (if only the maximum years of follow-up were reported, we would halve this value and add it to the mean age at baseline). For case–control studies, the age range associated with the OR estimate was simply the reported mean age at baseline (if mean age was not reported, we used the midpoint of the age range instead).
In the third step, we extracted age group-specific RR data and relevant bias covariates from the studies identified in our systematic review approach of age-specific smoking risk on CVD outcomes, and used MR-BRT to model the age pattern of excess risk (that is, RR-1) of smoking on CVD outcomes with age group-specific excess RR data for all CVD outcomes. We modeled the age pattern of smoking risk on CVDs following the same steps we implemented for modeling dose–response risk curves. In the final model, we included a spline on age, random slope on age by study and the bias covariate encoding exposure definition (that is, current, former and ever smokers), which was picked by the variable selection algorithm 28 , 29 . When predicting the age pattern of the excess risk of smoking on CVD outcomes using the fitted model, we did not include between-study heterogeneity to reduce uncertainty in the prediction.
In the fourth step, we calculated the age attenuation factors of excess risk compared with the reference age group for each CVD outcome as the ratio of the estimated excess risk for each age group to the excess risk for the reference age group. We performed the calculation at the draw level to obtain 1,000 draws of the age attenuation factors for each age group. Once we had estimated the age attenuation factors, we carried out the last step, which consisted of adjusting the risk curve for the reference age group from step 1 using equation (1) to produce the age group-specific risk curves for each CVD outcome:
We implemented the age adjustment at the draw level so that the uncertainty of the age attenuation factors could be naturally incorporated into the final adjusted age-specific RR curves. A PRISMA diagram detailing the systematic review approach, a description of the studies included and the full details about the methods are in Supplementary Information 1.5 and 5.2 .
Estimating the theoretical minimum risk exposure level
The theoretical minimum risk exposure level for smoking was 0, that is, no individuals in the population are current or former smokers.
Model validation
The validity of the meta-analytic tool has been extensively evaluated by Zheng and colleagues using simulation experiments 28 , 29 . For the present study, we conducted two additional sensitivity analyses to examine how the shape of the risk curves was impacted by applying a monotonicity constraint and trimming 10% of data. We present the results of these sensitivity analyses in Supplementary Information 6 . In addition to the sensitivity analyses, the dose–response risk estimates were also validated by plotting the mean risk function along with its 95% UI against both the extracted dose-specific RR data from the studies included and our previous dose–response risk estimates from the GBD 2019 (ref. 30 ). The mean risk functions along with the 95% UIs were validated based on data fit and the level, shape and plausibility of the dose–response risk curves. All curves were validated by all authors and reviewed by an external expert panel, comprising professors with relevant experience from universities including Johns Hopkins University, Karolinska Institute and University of Barcelona; senior scientists working in relevant departments at the WHO and the Center for Disease Control and Prevention (CDC) and directors of nongovernmental organizations such as the Campaign for Tobacco-Free Kids.
Statistical analysis
Analyses were carried out using R v.3.6.3, Python v.3.8 and Stata v.16.
Statistics and reproducibility
The study was a secondary analysis of existing data involving systematic reviews and meta-analyses. No statistical method was used to predetermine sample size. As the study did not involve primary data collection, randomization and blinding, data exclusions were not relevant to the present study, and, as such, no data were excluded and we performed no randomization or blinding. We have made our data and code available to foster reproducibility.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The findings from the present study are supported by data available in the published literature. Data sources and citations for each risk–outcome pair can be downloaded using the ‘download’ button on each risk curve page currently available at https://vizhub.healthdata.org/burden-of-proof . Study characteristics and citations for all input data used in the analyses are also provided in Supplementary Table 3 , and Supplementary Table 2 provides a template of the data collection form.
Code availability
All code used for these analyses is publicly available online ( https://github.com/ihmeuw-msca/burden-of-proof ).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung. Br. Med. J. 2 , 739–748 (1950).
Article CAS PubMed PubMed Central Google Scholar
Di Cicco, M. E., Ragazzo, V. & Jacinto, T. Mortality in relation to smoking: the British Doctors Study. Breathe 12 , 275–276 (2016).
Article PubMed PubMed Central Google Scholar
World Health Organization. WHO Framework Convention on Tobacco Control 36 (WHO, 2003).
Dai, X., Gakidou, E. & Lopez, A. D. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob. Control 31 , 129–137 (2022).
Article PubMed Google Scholar
Dikshit, R. P. & Kanhere, S. Tobacco habits and risk of lung, oropharyngeal and oral cavity cancer: a population-based case-control study in Bhopal, India. Int. J. Epidemiol. 29 , 609–614 (2000).
Article CAS PubMed Google Scholar
Liaw, K. M. & Chen, C. J. Mortality attributable to cigarette smoking in Taiwan: a 12-year follow-up study. Tob. Control 7 , 141–148 (1998).
Gandini, S. et al. Tobacco smoking and cancer: a meta-analysis. Int. J. Cancer 122 , 155–164 (2008).
Deng, X., Yuan, C. & Chang, D. Interactions between single nucleotide polymorphism of SERPINA1 gene and smoking in association with COPD: a case–control study. Int. J. Chron. Obstruct. Pulmon. Dis. 12 , 259–265 (2017).
Leem, A. Y., Park, B., Kim, Y. S., Jung, J. Y. & Won, S. Incidence and risk of chronic obstructive pulmonary disease in a Korean community-based cohort. Int. J. Chron. Obstruct. Pulmon. Dis. 13 , 509–517 (2018).
Forey, B. A., Thornton, A. J. & Lee, P. N. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulmon. Med. 11 , 36 (2011).
Article Google Scholar
Tan, J. et al. Smoking, blood pressure, and cardiovascular disease mortality in a large cohort of Chinese men with 15 years follow-up. Int. J. Environ. Res. Public Health 15 , E1026 (2018).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Br. Med. J. 328 , 1519 (2004).
Huxley, R. R. & Woodward, M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 378 , 1297–1305 (2011).
Hbejan, K. Smoking effect on ischemic heart disease in young patients. Heart Views 12 , 1–6 (2011).
Chao, H. et al. A meta-analysis of active smoking and risk of meningioma. Tob. Induc. Dis. 19 , 34 (2021).
Shi, H., Shao, X. & Hong, Y. Association between cigarette smoking and the susceptibility of acute myeloid leukemia: a systematic review and meta-analysis. Eur. Rev. Med Pharm. Sci. 23 , 10049–10057 (2019).
CAS Google Scholar
Macacu, A., Autier, P., Boniol, M. & Boyle, P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res. Treat. 154 , 213–224 (2015).
Pujades-Rodriguez, M. et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1 937 360 people in England: lifetime risks and implications for risk prediction. Int. J. Epidemiol. 44 , 129–141 (2015).
Kanazir, M. et al. Risk factors for hepatocellular carcinoma: a case-control study in Belgrade (Serbia). Tumori 96 , 911–917 (2010).
Pytynia, K. B. et al. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J. Clin. Oncol. 22 , 3981–3988 (2004).
Barengo, N. C., Antikainen, R., Harald, K. & Jousilahti, P. Smoking and cancer, cardiovascular and total mortality among older adults: the Finrisk Study. Prev. Med. Rep. 14 , 100875 (2019).
Guo, Y. et al. Modifiable risk factors for cognitive impairment in Parkinson’s disease: a systematic review and meta-analysis of prospective cohort studies. Mov. Disord. 34 , 876–883 (2019).
Aune, D., Vatten, L. J. & Boffetta, P. Tobacco smoking and the risk of gallbladder disease. Eur. J. Epidemiol. 31 , 643–653 (2016).
Qin, L., Deng, H.-Y., Chen, S.-J. & Wei, W. Relationship between cigarette smoking and risk of chronic myeloid leukaemia: a meta-analysis of epidemiological studies. Hematology 22 , 193–200 (2017).
Petrick, J. L. et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br. J. Cancer 118 , 1005–1012 (2018).
United States Department of Health, Education and Welfare. Smoking and Health. Report of the Advisory Committee on Smoking and Health to the Surgeon General of the United States Public Health Service https://www.cdc.gov/tobacco/data_statistics/sgr/index.htm (US DHEW, 1964).
United States Public Health Service Office of the Surgeon General & National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking Cessation: A Report of the Surgeon General . (US Department of Health and Human Services, 2020).
Zheng, P., Barber, R., Sorensen, R. J. D., Murray, C. J. L. & Aravkin, A. Y. Trimmed constrained mixed effects models: formulations and algorithms. J. Comput. Graph Stat. 30 , 544–556 (2021).
Zheng, P. et al. The Burden of Proof studies: assessing the evidence of risk. Nat. Med. in press (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br. Med. J. 339 , b2535 (2009).
Liu, Z. Y., He, X. Z. & Chapman, R. S. Smoking and other risk factors for lung cancer in Xuanwei, China. Int. J. Epidemiol. 20 , 26–31 (1991).
Brownson, R. C., Reif, J. S., Keefe, T. J., Ferguson, S. W. & Pritzl, J. A. Risk factors for adenocarcinoma of the lung. Am. J. Epidemiol. 125 , 25–34 (1987).
Marugame, T. et al. Lung cancer death rates by smoking status: comparison of the Three-Prefecture Cohort study in Japan to the Cancer Prevention Study II in the USA. Cancer Sci. 96 , 120–126 (2005).
Dosemeci, M., Gokmen, I., Unsal, M., Hayes, R. B. & Blair, A. Tobacco, alcohol use, and risks of laryngeal and lung cancer by subsite and histologic type in Turkey. Cancer Causes Control 8 , 729–737 (1997).
Freedman, N. D. et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int. J. Epidemiol. 45 , 846–856 (2016).
Bae, J.-M. et al. Lung cancer incidence by smoking status in Korean men: 16 years of observations in the Seoul Male Cancer Cohort study. J. Korean Med. Sci. 28 , 636–637 (2013).
Everatt, R., Kuzmickienė, I., Virvičiūtė, D. & Tamošiūnas, A. Cigarette smoking, educational level and total and site-specific cancer: a cohort study in men in Lithuania. Eur. J. Cancer Prev. 23 , 579–586 (2014).
Nordlund, L. A., Carstensen, J. M. & Pershagen, G. Are male and female smokers at equal risk of smoking-related cancer: evidence from a Swedish prospective study. Scand. J. Public Health 27 , 56–62 (1999).
Siemiatycki, J., Krewski, D., Franco, E. & Kaiserman, M. Associations between cigarette smoking and each of 21 types of cancer: a multi-site case–control study. Int. J. Epidemiol. 24 , 504–514 (1995).
Chyou, P. H., Nomura, A. M. & Stemmermann, G. N. A prospective study of the attributable risk of cancer due to cigarette smoking. Am. J. Public Health 82 , 37–40 (1992).
Potter, J. D., Sellers, T. A., Folsom, A. R. & McGovern, P. G. Alcohol, beer, and lung cancer in postmenopausal women. The Iowa Women’s Health Study. Ann. Epidemiol. 2 , 587–595 (1992).
Chyou, P. H., Nomura, A. M., Stemmermann, G. N. & Kato, I. Lung cancer: a prospective study of smoking, occupation, and nutrient intake. Arch. Environ. Health 48 , 69–72 (1993).
Pesch, B. et al. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int. J. Cancer 131 , 1210–1219 (2012).
Jöckel, K. H. et al. Occupational and environmental hazards associated with lung cancer. Int. J. Epidemiol. 21 , 202–213 (1992).
Jöckel, K. H., Ahrens, W., Jahn, I., Pohlabeln, H. & Bolm-Audorff, U. Occupational risk factors for lung cancer: a case-control study in West Germany. Int. J. Epidemiol. 27 , 549–560 (1998).
Lei, Y. X., Cai, W. C., Chen, Y. Z. & Du, Y. X. Some lifestyle factors in human lung cancer: a case-control study of 792 lung cancer cases. Lung Cancer 14 , S121–S136 (1996).
Pawlega, J., Rachtan, J. & Dyba, T. Evaluation of certain risk factors for lung cancer in Cracow (Poland)—a case–control study. Acta Oncol. 36 , 471–476 (1997).
Mao, Y. et al. Socioeconomic status and lung cancer risk in Canada. Int. J. Epidemiol. 30 , 809–817 (2001).
Barbone, F., Bovenzi, M., Cavallieri, F. & Stanta, G. Cigarette smoking and histologic type of lung cancer in men. Chest 112 , 1474–1479 (1997).
Matos, E., Vilensky, M., Boffetta, P. & Kogevinas, M. Lung cancer and smoking: a case–control study in Buenos Aires, Argentina. Lung Cancer 21 , 155–163 (1998).
Simonato, L. et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int. J. Cancer 91 , 876–887 (2001).
Risch, H. A. et al. Are female smokers at higher risk for lung cancer than male smokers? A case–control analysis by histologic type. Am. J. Epidemiol. 138 , 281–293 (1993).
Sankaranarayanan, R. et al. A case–control study of diet and lung cancer in Kerala, south India. Int. J. Cancer 58 , 644–649 (1994).
Band, P. R. et al. Identification of occupational cancer risks in British Columbia. Part I: methodology, descriptive results, and analysis of cancer risks, by cigarette smoking categories of 15,463 incident cancer cases. J. Occup. Environ. Med. 41 , 224–232 (1999).
Becher, H., Jöckel, K. H., Timm, J., Wichmann, H. E. & Drescher, K. Smoking cessation and nonsmoking intervals: effect of different smoking patterns on lung cancer risk. Cancer Causes Control 2 , 381–387 (1991).
Brockmöller, J., Kerb, R., Drakoulis, N., Nitz, M. & Roots, I. Genotype and phenotype of glutathione S-transferase class mu isoenzymes mu and psi in lung cancer patients and controls. Cancer Res. 53 , 1004–1011 (1993).
PubMed Google Scholar
Vena, J. E., Byers, T. E., Cookfair, D. & Swanson, M. Occupation and lung cancer risk. An analysis by histologic subtypes. Cancer 56 , 910–917 (1985).
Cascorbi, I. et al. Homozygous rapid arylamine N -acetyltransferase (NAT2) genotype as a susceptibility factor for lung cancer. Cancer Res. 56 , 3961–3966 (1996).
CAS PubMed Google Scholar
Chiazze, L., Watkins, D. K. & Fryar, C. A case–control study of malignant and non-malignant respiratory disease among employees of a fiberglass manufacturing facility. Br. J. Ind. Med 49 , 326–331 (1992).
CAS PubMed PubMed Central Google Scholar
Ando, M. et al. Attributable and absolute risk of lung cancer death by smoking status: findings from the Japan Collaborative Cohort Study. Int. J. Cancer 105 , 249–254 (2003).
De Matteis, S. et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am. J. Epidemiol. 177 , 601–612 (2013).
He, Y. et al. Changes in smoking behavior and subsequent mortality risk during a 35-year follow-up of a cohort in Xi’an, China. Am. J. Epidemiol. 179 , 1060–1070 (2014).
Nishino, Y. et al. Cancer incidence profiles in the Miyagi Cohort Study. J. Epidemiol. 14 , S7–S11 (2004).
Papadopoulos, A. et al. Cigarette smoking and lung cancer in women: results of the French ICARE case–control study. Lung Cancer 74 , 369–377 (2011).
Shimazu, T. et al. Alcohol and risk of lung cancer among Japanese men: data from a large-scale population-based cohort study, the JPHC study. Cancer Causes Control 19 , 1095–1102 (2008).
Tindle, H. A. et al. Lifetime smoking history and risk of lung cancer: results from the Framingham Heart Study. J. Natl Cancer Inst. 110 , 1201–1207 (2018).
PubMed PubMed Central Google Scholar
Yong, L. C. et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 146 , 231–243 (1997).
Hansen, M. S. et al. Sex differences in risk of smoking-associated lung cancer: results from a cohort of 600,000 Norwegians. Am. J. Epidemiol. 187 , 971–981 (2018).
Boffetta, P. et al. Tobacco smoking as a risk factor of bronchioloalveolar carcinoma of the lung: pooled analysis of seven case-control studies in the International Lung Cancer Consortium (ILCCO). Cancer Causes Control 22 , 73–79 (2011).
Yun, Y. D. et al. Hazard ratio of smoking on lung cancer in Korea according to histological type and gender. Lung 194 , 281–289 (2016).
Suzuki, I. et al. Risk factors for lung cancer in Rio de Janeiro, Brazil: a case–control study. Lung Cancer 11 , 179–190 (1994).
De Stefani, E., Deneo-Pellegrini, H., Carzoglio, J. C., Ronco, A. & Mendilaharsu, M. Dietary nitrosodimethylamine and the risk of lung cancer: a case–control study from Uruguay. Cancer Epidemiol. Biomark. Prev. 5 , 679–682 (1996).
Google Scholar
Kreuzer, M. et al. Risk factors for lung cancer in young adults. Am. J. Epidemiol. 147 , 1028–1037 (1998).
Armadans-Gil, L., Vaqué-Rafart, J., Rosselló, J., Olona, M. & Alseda, M. Cigarette smoking and male lung cancer risk with special regard to type of tobacco. Int. J. Epidemiol. 28 , 614–619 (1999).
Kubík, A. K., Zatloukal, P., Tomásek, L. & Petruzelka, L. Lung cancer risk among Czech women: a case–control study. Prev. Med. 34 , 436–444 (2002).
Rachtan, J. Smoking, passive smoking and lung cancer cell types among women in Poland. Lung Cancer 35 , 129–136 (2002).
Thun, M. J. et al. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 368 , 351–364 (2013).
Zatloukal, P., Kubík, A., Pauk, N., Tomásek, L. & Petruzelka, L. Adenocarcinoma of the lung among women: risk associated with smoking, prior lung disease, diet and menstrual and pregnancy history. Lung Cancer 41 , 283–293 (2003).
Hansen, M. S., Licaj, I., Braaten, T., Lund, E. & Gram, I. T. The fraction of lung cancer attributable to smoking in the Norwegian Women and Cancer (NOWAC) Study. Br. J. Cancer 124 , 658–662 (2021).
Zhang, P. et al. Association of smoking and polygenic risk with the incidence of lung cancer: a prospective cohort study. Br. J. Cancer 126 , 1637–1646 (2022).
Weber, M. F. et al. Cancer incidence and cancer death in relation to tobacco smoking in a population-based Australian cohort study. Int. J. Cancer 149 , 1076–1088 (2021).
Guo, L.-W. et al. A risk prediction model for selecting high-risk population for computed tomography lung cancer screening in China. Lung Cancer 163 , 27–34 (2022).
Mezzoiuso, A. G., Odone, A., Signorelli, C. & Russo, A. G. Association between smoking and cancers among women: results from the FRiCaM multisite cohort study. J. Cancer 12 , 3136–3144 (2021).
Hawrysz, I., Wadolowska, L., Slowinska, M. A., Czerwinska, A. & Golota, J. J. Adherence to prudent and mediterranean dietary patterns is inversely associated with lung cancer in moderate but not heavy male Polish smokers: a case–control study. Nutrients 12 , E3788 (2020).
Huang, C.-C., Lai, C.-Y., Tsai, C.-H., Wang, J.-Y. & Wong, R.-H. Combined effects of cigarette smoking, DNA methyltransferase 3B genetic polymorphism, and DNA damage on lung cancer. BMC Cancer 21 , 1066 (2021).
Viner, B., Barberio, A. M., Haig, T. R., Friedenreich, C. M. & Brenner, D. R. The individual and combined effects of alcohol consumption and cigarette smoking on site-specific cancer risk in a prospective cohort of 26,607 adults: results from Alberta’s Tomorrow Project. Cancer Causes Control 30 , 1313–1326 (2019).
Park, E. Y., Lim, M. K., Park, E., Oh, J.-K. & Lee, D.-H. Relationship between urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and lung cancer risk in the general population: a community-based prospective cohort study. Front. Oncol. 11 , 611674 (2021).
De Stefani, E., Deneo-Pellegrini, H., Mendilaharsu, M., Carzoglio, J. C. & Ronco, A. Dietary fat and lung cancer: a case–control study in Uruguay. Cancer Causes Control 8 , 913–921 (1997).
Wünsch-Filho, V., Moncau, J. E., Mirabelli, D. & Boffetta, P. Occupational risk factors of lung cancer in São Paulo, Brazil. Scand. J. Work Environ. Health 24 , 118–124 (1998).
Hu, J. et al. A case-control study of diet and lung cancer in northeast China. Int. J. Cancer 71 , 924–931 (1997).
Jia, G., Wen, W., Massion, P. P., Shu, X.-O. & Zheng, W. Incorporating both genetic and tobacco smoking data to identify high-risk smokers for lung cancer screening. Carcinogenesis 42 , 874–879 (2021).
Rusmaully, J. et al. Risk of lung cancer among women in relation to lifetime history of tobacco smoking: a population-based case–control study in France (the WELCA study). BMC Cancer 21 , 711 (2021).
Jin, K. et al. Tobacco smoking modifies the association between hormonal factors and lung cancer occurrence among post-menopausal Chinese women. Transl. Oncol. 12 , 819–827 (2019).
Tse, L. A., Wang, F., Wong, M. C.-S., Au, J. S.-K. & Yu, I. T.-S. Risk assessment and prediction for lung cancer among Hong Kong Chinese men. BMC Cancer 22 , 585 (2022).
Huang, C.-C. et al. Joint effects of cigarette smoking and green tea consumption with miR-29b and DNMT3b mRNA expression in the development of lung cancer. Genes 13 , 836 (2022).
Hosseini, M. et al. Environmental risk factors for lung cancer in Iran: a case–control study. Int. J. Epidemiol. 38 , 989–996 (2009).
Naghibzadeh-Tahami, A. et al. Is opium use associated with an increased risk of lung cancer? A case–control study. BMC Cancer 20 , 807 (2020).
Shimatani, K., Ito, H., Matsuo, K., Tajima, K. & Takezaki, T. Cumulative cigarette tar exposure and lung cancer risk among Japanese smokers. Jpn J. Clin. Oncol. 50 , 1009–1017 (2020).
Lai, C.-Y. et al. Genetic polymorphism of catechol- O -methyltransferase modulates the association of green tea consumption and lung cancer. Eur. J. Cancer Prev. 28 , 316–322 (2019).
Schwartz, A. G. et al. Hormone use, reproductive history, and risk of lung cancer: the Women’s Health Initiative studies. J. Thorac. Oncol. 10 , 1004–1013 (2015).
Kreuzer, M., Gerken, M., Heinrich, J., Kreienbrock, L. & Wichmann, H.-E. Hormonal factors and risk of lung cancer among women? Int. J. Epidemiol. 32 , 263–271 (2003).
Sreeja, L. et al. Possible risk modification by CYP1A1, GSTM1 and GSTT1 gene polymorphisms in lung cancer susceptibility in a South Indian population. J. Hum. Genet. 50 , 618–627 (2005).
Siemiatycki, J. et al. Are the apparent effects of cigarette smoking on lung and bladder cancers due to uncontrolled confounding by occupational exposures? Epidemiology 5 , 57–65 (1994).
Chan-Yeung, M. et al. Risk factors associated with lung cancer in Hong Kong. Lung Cancer 40 , 131–140 (2003).
Lawania, S., Singh, N., Behera, D. & Sharma, S. Xeroderma pigmentosum complementation group D polymorphism toward lung cancer susceptibility survival and response in patients treated with platinum chemotherapy. Future Oncol. 13 , 2645–2665 (2017).
De Stefani, E. et al. Mate drinking and risk of lung cancer in males: a case-control study from Uruguay. Cancer Epidemiol. Biomark. Prev. 5 , 515–519 (1996).
Pérez-Padilla, R. et al. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am. J. Respir. Crit. Care Med. 154 , 701–706 (1996).
Zhang, X.-R. et al. Glucosamine use, smoking and risk of incident chronic obstructive pulmonary disease: a large prospective cohort study. Br. J. Nutr . https://doi.org/10.1017/S000711452100372X (2021).
Johannessen, A., Omenaas, E., Bakke, P. & Gulsvik, A. Incidence of GOLD-defined chronic obstructive pulmonary disease in a general adult population. Int. J. Tuberc. Lung Dis. 9 , 926–932 (2005).
Fox, J. Life-style and mortality: a large-scale census-based cohort study in Japan. J. Epidemiol. Community Health 45 , 173 (1991).
Article PubMed Central Google Scholar
Thomson, B. et al. Low-intensity daily smoking and cause-specific mortality in Mexico: prospective study of 150 000 adults. Int. J. Epidemiol. 50 , 955–964 (2021).
van Durme, Y. M. T. A. et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest 135 , 368–377 (2009).
Li, L. et al. SERPINE2 rs16865421 polymorphism is associated with a lower risk of chronic obstructive pulmonary disease in the Uygur population: a case–control study. J. Gene Med. 21 , e3106 (2019).
Ganbold, C. et al. The cumulative effect of gene-gene interactions between GSTM1 , CHRNA3 , CHRNA5 and SOD3 gene polymorphisms combined with smoking on COPD risk. Int. J. Chron. Obstruct. Pulmon. Dis. 16 , 2857–2868 (2021).
Omori, H. et al. Twelve-year cumulative incidence of airflow obstruction among Japanese males. Intern. Med. 50 , 1537–1544 (2011).
Manson, J. E., Ajani, U. A., Liu, S., Nathan, D. M. & Hennekens, C. H. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am. J. Med. 109 , 538–542 (2000).
Lv, J. et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int. J. Epidemiol. 46 , 1410–1420 (2017).
Waki, K. et al. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet. Med. 22 , 323–331 (2005).
Meisinger, C., Döring, A., Thorand, B. & Löwel, H. Association of cigarette smoking and tar and nicotine intake with development of type 2 diabetes mellitus in men and women from the general population: the MONICA/KORA Augsburg Cohort Study. Diabetologia 49 , 1770–1776 (2006).
Huh, Y. et al. Association of smoking status with the risk of type 2 diabetes among young adults: a nationwide cohort study in South Korea. Nicotine Tob. Res. 24 , 1234–1240 (2022).
Sawada, S. S., Lee, I.-M., Muto, T., Matuszaki, K. & Blair, S. N. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care 26 , 2918–2922 (2003).
Will, J. C., Galuska, D. A., Ford, E. S., Mokdad, A. & Calle, E. E. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int. J. Epidemiol. 30 , 540–546 (2001).
Nakanishi, N., Nakamura, K., Matsuo, Y., Suzuki, K. & Tatara, K. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann. Intern. Med. 133 , 183–191 (2000).
Sairenchi, T. et al. Cigarette smoking and risk of type 2 diabetes mellitus among middle-aged and elderly Japanese men and women. Am. J. Epidemiol. 160 , 158–162 (2004).
Hou, X. et al. Cigarette smoking is associated with a lower prevalence of newly diagnosed diabetes screened by OGTT than non-smoking in Chinese men with normal weight. PLoS ONE 11 , e0149234 (2016).
Hu, F. B. et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345 , 790–797 (2001).
Teratani, T. et al. Dose-response relationship between tobacco or alcohol consumption and the development of diabetes mellitus in Japanese male workers. Drug Alcohol Depend. 125 , 276–282 (2012).
Kawakami, N., Takatsuka, N., Shimizu, H. & Ishibashi, H. Effects of smoking on the incidence of non-insulin-dependent diabetes mellitus. Replication and extension in a Japanese cohort of male employees. Am. J. Epidemiol. 145 , 103–109 (1997).
Patja, K. et al. Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J. Intern. Med. 258 , 356–362 (2005).
White, W. B. et al. High-intensity cigarette smoking is associated with incident diabetes mellitus in Black adults: the Jackson Heart Study. J. Am. Heart Assoc. 7 , e007413 (2018).
Uchimoto, S. et al. Impact of cigarette smoking on the incidence of Type 2 diabetes mellitus in middle-aged Japanese men: the Osaka Health Survey. Diabet. Med . 16 , 951–955 (1999).
Rimm, E. B., Chan, J., Stampfer, M. J., Colditz, G. A. & Willett, W. C. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. Br. Med. J. 310 , 555–559 (1995).
Article CAS Google Scholar
Hilawe, E. H. et al. Smoking and diabetes: is the association mediated by adiponectin, leptin, or C-reactive protein? J. Epidemiol. 25 , 99–109 (2015).
InterAct, Consortium et al. Smoking and long-term risk of type 2 diabetes: the EPIC-InterAct study in European populations. Diabetes Care 37 , 3164–3171 (2014).
Jee, S. H., Foong, A. W., Hur, N. W. & Samet, J. M. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care 33 , 2567–2572 (2010).
Rasouli, B. et al. Smoking and the risk of LADA: results from a Swedish population-based case-control study. Diabetes Care 39 , 794–800 (2016).
Wannamethee, S. G., Shaper, A. G. & Perry, I. J., British Regional Heart Study. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care 24 , 1590–1595 (2001).
Radzeviciene, L. & Ostrauskas, R. Smoking habits and type 2 diabetes mellitus in women. Women Health 58 , 884–897 (2018).
Carlsson, S., Midthjell, K. & Grill, V., Nord-Trøndelag Study. Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: an 11-year follow-up of incidence of diabetes in the Nord-Trøndelag study. Diabetologia 47 , 1953–1956 (2004).
Akter, S. et al. Smoking, smoking cessation, and the risk of type 2 diabetes among Japanese adults: Japan Epidemiology Collaboration on Occupational Health Study. PLoS ONE 10 , e0132166 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Park, C.-H. et al. [The effect of smoking status upon occurrence of impaired fasting glucose or type 2 diabetes in Korean men]. J. Prev. Med. Public Health 41 , 249–254 (2008).
Doi, Y. et al. Two risk score models for predicting incident Type 2 diabetes in Japan. Diabet. Med. 29 , 107–114 (2012).
van den Brandt, P. A. A possible dual effect of cigarette smoking on the risk of postmenopausal breast cancer. Eur. J. Epidemiol. 32 , 683–690 (2017).
Dossus, L. et al. Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int. J. Cancer 134 , 1871–1888 (2014).
Kawai, M., Malone, K. E., Tang, M.-T. C. & Li, C. I. Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer 120 , 1026–1034 (2014).
Reynolds, P. et al. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J. Natl Cancer Inst. 96 , 29–37 (2004).
Ellingjord-Dale, M. et al. Alcohol, physical activity, smoking, and breast cancer subtypes in a large, nested case-control study from the Norwegian Breast Cancer Screening Program. Cancer Epidemiol. Biomark. Prev. 26 , 1736–1744 (2017).
Arthur, R. et al. Association between lifestyle, menstrual/reproductive history, and histological factors and risk of breast cancer in women biopsied for benign breast disease. Breast Cancer Res. Treat. 165 , 623–631 (2017).
Luo, J. et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. Br. Med. J. 342 , d1016 (2011).
White, A. J., D’Aloisio, A. A., Nichols, H. B., DeRoo, L. A. & Sandler, D. P. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control 28 , 667–675 (2017).
Gram, I. T. et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol. Biomark. Prev. 14 , 61–66 (2005).
Gammon, M. D. et al. Cigarette smoking and breast cancer risk among young women (United States). Cancer Causes Control 9 , 583–590 (1998).
Magnusson, C., Wedrén, S. & Rosenberg, L. U. Cigarette smoking and breast cancer risk: a population-based study in Sweden. Br. J. Cancer 97 , 1287–1290 (2007).
Chu, S. Y. et al. Cigarette smoking and the risk of breast cancer. Am. J. Epidemiol. 131 , 244–253 (1990).
Lemogne, C. et al. Depression and the risk of cancer: a 15-year follow-up study of the GAZEL cohort. Am. J. Epidemiol. 178 , 1712–1720 (2013).
Morabia, A., Bernstein, M., Héritier, S. & Khatchatrian, N. Relation of breast cancer with passive and active exposure to tobacco smoke. Am. J. Epidemiol. 143 , 918–928 (1996).
Conlon, M. S. C., Johnson, K. C., Bewick, M. A., Lafrenie, R. M. & Donner, A. Smoking (active and passive), N -acetyltransferase 2, and risk of breast cancer. Cancer Epidemiol. 34 , 142–149 (2010).
Ozasa, K., Japan Collaborative Cohort Study for Evaluation of Cancer. Smoking and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac. J. Cancer Prev. 8 , 89–96 (2007).
Jones, M. E., Schoemaker, M. J., Wright, L. B., Ashworth, A. & Swerdlow, A. J. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 19 , 118 (2017).
Bjerkaas, E. et al. Smoking duration before first childbirth: an emerging risk factor for breast cancer? Results from 302,865 Norwegian women. Cancer Causes Control 24 , 1347–1356 (2013).
Gram, I. T., Little, M. A., Lund, E. & Braaten, T. The fraction of breast cancer attributable to smoking: the Norwegian women and cancer study 1991–2012. Br. J. Cancer 115 , 616–623 (2016).
Li, C. I., Malone, K. E. & Daling, J. R. The relationship between various measures of cigarette smoking and risk of breast cancer among older women 65–79 years of age (United States). Cancer Causes Control 16 , 975–985 (2005).
Xue, F., Willett, W. C., Rosner, B. A., Hankinson, S. E. & Michels, K. B. Cigarette smoking and the incidence of breast cancer. Arch. Intern. Med. 171 , 125–133 (2011).
Parker, A. S., Cerhan, J. R., Putnam, S. D., Cantor, K. P. & Lynch, C. F. A cohort study of farming and risk of prostate cancer in Iowa. Epidemiology 10 , 452–455 (1999).
Sawada, N. et al. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center-based prospective study. Int. J. Cancer 134 , 971–978 (2014).
Hiatt, R. A., Armstrong, M. A., Klatsky, A. L. & Sidney, S. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control 5 , 66–72 (1994).
Cerhan, J. R. et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 8 , 229–238 (1997).
Watters, J. L., Park, Y., Hollenbeck, A., Schatzkin, A. & Albanes, D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol. Biomark. Prev. 18 , 2427–2435 (2009).
Butler, L. M., Wang, R., Wong, A. S., Koh, W.-P. & Yu, M. C. Cigarette smoking and risk of prostate cancer among Singapore Chinese. Cancer Causes Control 20 , 1967–1974 (2009).
Lotufo, P. A., Lee, I. M., Ajani, U. A., Hennekens, C. H. & Manson, J. E. Cigarette smoking and risk of prostate cancer in the physicians’ health study (United States). Int. J. Cancer 87 , 141–144 (2000).
Hsing, A. W. et al. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 50 , 6836–6840 (1990).
Veierød, M. B., Laake, P. & Thelle, D. S. Dietary fat intake and risk of prostate cancer: a prospective study of 25,708 Norwegian men. Int. J. Cancer 73 , 634–638 (1997).
Meyer, J., Rohrmann, S., Bopp, M. & Faeh, D. & Swiss National Cohort Study Group. Impact of smoking and excess body weight on overall and site-specific cancer mortality risk. Cancer Epidemiol. Biomark. Prev . 24 , 1516–1522 (2015).
Putnam, S. D. et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann. Epidemiol. 10 , 361–369 (2000).
Taghizadeh, N., Vonk, J. M. & Boezen, H. M. Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of follow-up. PLoS ONE 11 , e0153310 (2016).
Park, S.-Y. et al. Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: the Multiethnic Cohort Study. Cancer Causes Control 26 , 1507–1515 (2015).
Nomura, A. M., Lee, J., Stemmermann, G. N. & Combs, G. F. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 9 , 883–887 (2000).
Rodriguez, C., Tatham, L. M., Thun, M. J., Calle, E. E. & Heath, C. W. Smoking and fatal prostate cancer in a large cohort of adult men. Am. J. Epidemiol. 145 , 466–475 (1997).
Rohrmann, S. et al. Smoking and risk of fatal prostate cancer in a prospective U.S. study. Urology 69 , 721–725 (2007).
Giovannucci, E. et al. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol. Biomark. Prev. 8 , 277–282 (1999).
Rohrmann, S. et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 108 , 708–714 (2013).
Lund Nilsen, T. I., Johnsen, R. & Vatten, L. J. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br. J. Cancer 82 , 1358–1363 (2000).
Hsing, A. W., McLaughlin, J. K., Hrubec, Z., Blot, W. J. & Fraumeni, J. F. Tobacco use and prostate cancer: 26-year follow-up of US veterans. Am. J. Epidemiol. 133 , 437–441 (1991).
Murray, C. J. L. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1223–1249 (2020).
Bero, L. A. & Jadad, A. R. How consumers and policymakers can use systematic reviews for decision making. Ann. Intern. Med. 127 , 37–42 (1997).
Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb. Mortal. Wkly Rep. 58 , 1227–1232 (2009).
Prochaska, J. O. & Goldstein, M. G. Process of smoking cessation: implications for clinicians. Clin. Chest Med. 12 , 727–735 (1991).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br. Med. J. 372 , n71 (2021).
Stevens, G. A. et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet 388 , e19–e23 (2016).
BMJ Best Practice. What is GRADE? https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade (BMJ, 2021).
The GRADE Working Group. GRADE handbook . https://gdt.gradepro.org/app/handbook/handbook.html (The GRADE Working Group, 2013).
Efron, B., Hastie, T., Johnstone, I. & Tibshirani, R. Least angle regression. Ann. Stat. 32 , 407–499 (2004).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 58 , 267–288 (1996).
von Hippel, P. T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 15 , 35 (2015).
Kontopantelis, E., Springate, D. A. & Reeves, D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS ONE 8 , e69930 (2013).
Biggerstaff, B. J. & Tweedie, R. L. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat. Med. 16 , 753–768 (1997).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315 , 629–634 (1997).
Lee, P. N., Forey, B. A. & Coombs, K. J. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 12 , 385 (2012).
Rücker, G., Carpenter, J. R. & Schwarzer, G. Detecting and adjusting for small-study effects in meta-analysis. Biometr. J. 53 , 351–368 (2011).
Wu, Z.-J., Zhao, P., Liu, B. & Yuan, Z.-C. Effect of cigarette smoking on risk of hip fracture in men: a meta-analysis of 14 prospective cohort studies. PLoS ONE 11 , e0168990 (2016).
Thun, M. J. et al. in Cigarette Smoking Behaviour in the United States: changes in cigarette-related disease risks and their implication for prevention and control (eds Burns, D.M. et al.) Tobacco Control Monograph No. 8 Ch. 4 (National Cancer Institute, 1997).
Tolstrup, J. S. et al. Smoking and risk of coronary heart disease in younger, middle-aged, and older adults. Am. J. Public Health 104 , 96–102 (2014).
Jonas, M. A., Oates, J. A., Ockene, J. K. & Hennekens, C. H. Statement on smoking and cardiovascular disease for health care professionals. American Heart Association. Circulation 86 , 1664–1669 (1992).
Khan, S. S. et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J. Am. Heart Assoc. 10 , e021751 (2021).
Download references
Acknowledgements
Research reported in this publication was supported by the Bill & Melinda Gates Foundation and Bloomberg Philanthropies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The study funders had no role in study design, data collection, data analysis, data interpretation, writing of the final report or the decision to publish.
We thank the Tobacco Metrics Team Advisory Group for their valuable input and review of the work. The members of the Advisory Group are: P. Allebeck, R. Chandora, J. Drope, M. Eriksen, E. Fernández, H. Gouda, R. Kennedy, D. McGoldrick, L. Pan, K. Schotte, E. Sebrie, J. Soriano, M. Tynan and K. Welding.
Author information
Authors and affiliations.
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
Xiaochen Dai, Gabriela F. Gil, Marissa B. Reitsma, Noah S. Ahmad, Jason A. Anderson, Catherine Bisignano, Sinclair Carr, Rachel Feldman, Simon I. Hay, Jiawei He, Vincent Iannucci, Hilary R. Lawlor, Matthew J. Malloy, Laurie B. Marczak, Susan A. McLaughlin, Larissa Morikawa, Erin C. Mullany, Sneha I. Nicholson, Erin M. O’Connell, Chukwuma Okereke, Reed J. D. Sorensen, Joanna Whisnant, Aleksandr Y. Aravkin, Peng Zheng, Christopher J. L. Murray & Emmanuela Gakidou
Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA
Xiaochen Dai, Simon I. Hay, Jiawei He, Peng Zheng, Christopher J. L. Murray & Emmanuela Gakidou
Department of Applied Mathematics, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin
You can also search for this author in PubMed Google Scholar
Contributions
X.D., S.I.H., S.A.M., E.C.M., E.M.O., C.J.L.M. and E.G. managed the estimation or publications process. X.D. and G.F.G. wrote the first draft of the manuscript. X.D. and P.Z. had primary responsibility for applying analytical methods to produce estimates. X.D., G.F.G., N.S.A., J.A.A., S.C., R.F., V.I., M.J.M., L.M., S.I.N., C.O., M.B.R. and J.W. had primary responsibility for seeking, cataloguing, extracting or cleaning data, and for designing or coding figures and tables. X.D., G.F.G., M.B.R., N.S.A., H.R.L., C.O. and J.W. provided data or critical feedback on data sources. X.D., J.H., R.J.D.S., A.Y.A., P.Z., C.J.L.M. and E.G. developed methods or computational machinery. X.D., G.F.G., M.B.R., S.I.H., J.H., R.J.D.S., A.Y.A., P.Z., C.J.L.M. and E.G. provided critical feedback on methods or results. X.D., G.F.G., M.B.R., C.B., S.I.H., L.B.M., S.A.M., A.Y.A. and E.G. drafted the work or revised it critically for important intellectual content. X.D., S.I.H., L.B.M., E.C.M., E.M.O. and E.G. managed the overall research enterprise.
Corresponding author
Correspondence to Xiaochen Dai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Frederic Sitas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jennifer Sargent and Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 prisma 2020 flow diagram for an updated systematic review of the smoking and tracheal, bronchus, and lung cancer risk-outcome pair..
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and lung cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 2 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Chronic obstructive pulmonary disease risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and chronic obstructive pulmonary disease conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 3 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Diabetes mellitus type 2 risk- outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and type 2 diabetes conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 4 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Breast cancer risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and breast cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 5 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Prostate cancer risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and prostate cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 6 Smoking and Breast Cancer.
a , log-relative risk function. b , relative risk function. c , A modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard deviation (SD) that includes reported SD and between-study heterogeneity on the y-axis.
Supplementary information
Supplementary information.
Supplementary Information 1: Data source identification and assessment. Supplementary Information 2: Data inputs. Supplementary Information 3: Study quality and bias assessment. Supplementary Information 4: The dose–response RR curves and their 95% UIs for all smoking–outcome pairs. Supplementary Information 5: Supplementary methods. Supplementary Information 6: Sensitivity analysis. Supplementary Information 7: Binary smoking–outcome pair. Supplementary Information 8: Risk curve details. Supplementary Information 9: GATHER and PRISMA checklists.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Dai, X., Gil, G.F., Reitsma, M.B. et al. Health effects associated with smoking: a Burden of Proof study. Nat Med 28 , 2045–2055 (2022). https://doi.org/10.1038/s41591-022-01978-x
Download citation
Received : 11 April 2022
Accepted : 28 July 2022
Published : 10 October 2022
Issue Date : October 2022
DOI : https://doi.org/10.1038/s41591-022-01978-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Environmental and Health Impacts of Air Pollution: A Review
Ioannis manisalidis, elisavet stavropoulou, agathangelos stavropoulos, eugenia bezirtzoglou.
- Author information
- Article notes
- Copyright and License information
Edited by: Ethel Eljarrat, Institute of Environmental Assessment and Water Research (CSIC), Spain
Reviewed by: Fei Li, Zhongnan University of Economics and Law, China; M. Jahangir Alam, University of Houston, United States
*Correspondence: Ioannis Manisalidis [email protected]
Elisavet Stavropoulou [email protected]
This article was submitted to Environmental Health, a section of the journal Frontiers in Public Health
†These authors have contributed equally to this work
Received 2019 Oct 17; Accepted 2020 Jan 17; Collection date 2020.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
One of our era's greatest scourges is air pollution, on account not only of its impact on climate change but also its impact on public and individual health due to increasing morbidity and mortality. There are many pollutants that are major factors in disease in humans. Among them, Particulate Matter (PM), particles of variable but very small diameter, penetrate the respiratory system via inhalation, causing respiratory and cardiovascular diseases, reproductive and central nervous system dysfunctions, and cancer. Despite the fact that ozone in the stratosphere plays a protective role against ultraviolet irradiation, it is harmful when in high concentration at ground level, also affecting the respiratory and cardiovascular system. Furthermore, nitrogen oxide, sulfur dioxide, Volatile Organic Compounds (VOCs), dioxins, and polycyclic aromatic hydrocarbons (PAHs) are all considered air pollutants that are harmful to humans. Carbon monoxide can even provoke direct poisoning when breathed in at high levels. Heavy metals such as lead, when absorbed into the human body, can lead to direct poisoning or chronic intoxication, depending on exposure. Diseases occurring from the aforementioned substances include principally respiratory problems such as Chronic Obstructive Pulmonary Disease (COPD), asthma, bronchiolitis, and also lung cancer, cardiovascular events, central nervous system dysfunctions, and cutaneous diseases. Last but not least, climate change resulting from environmental pollution affects the geographical distribution of many infectious diseases, as do natural disasters. The only way to tackle this problem is through public awareness coupled with a multidisciplinary approach by scientific experts; national and international organizations must address the emergence of this threat and propose sustainable solutions.
Keywords: air pollution, environment, health, public health, gas emission, policy
Approach to the Problem
The interactions between humans and their physical surroundings have been extensively studied, as multiple human activities influence the environment. The environment is a coupling of the biotic (living organisms and microorganisms) and the abiotic (hydrosphere, lithosphere, and atmosphere).
Pollution is defined as the introduction into the environment of substances harmful to humans and other living organisms. Pollutants are harmful solids, liquids, or gases produced in higher than usual concentrations that reduce the quality of our environment.
Human activities have an adverse effect on the environment by polluting the water we drink, the air we breathe, and the soil in which plants grow. Although the industrial revolution was a great success in terms of technology, society, and the provision of multiple services, it also introduced the production of huge quantities of pollutants emitted into the air that are harmful to human health. Without any doubt, the global environmental pollution is considered an international public health issue with multiple facets. Social, economic, and legislative concerns and lifestyle habits are related to this major problem. Clearly, urbanization and industrialization are reaching unprecedented and upsetting proportions worldwide in our era. Anthropogenic air pollution is one of the biggest public health hazards worldwide, given that it accounts for about 9 million deaths per year ( 1 ).
Without a doubt, all of the aforementioned are closely associated with climate change, and in the event of danger, the consequences can be severe for mankind ( 2 ). Climate changes and the effects of global planetary warming seriously affect multiple ecosystems, causing problems such as food safety issues, ice and iceberg melting, animal extinction, and damage to plants ( 3 , 4 ).
Air pollution has various health effects. The health of susceptible and sensitive individuals can be impacted even on low air pollution days. Short-term exposure to air pollutants is closely related to COPD (Chronic Obstructive Pulmonary Disease), cough, shortness of breath, wheezing, asthma, respiratory disease, and high rates of hospitalization (a measurement of morbidity).
The long-term effects associated with air pollution are chronic asthma, pulmonary insufficiency, cardiovascular diseases, and cardiovascular mortality. According to a Swedish cohort study, diabetes seems to be induced after long-term air pollution exposure ( 5 ). Moreover, air pollution seems to have various malign health effects in early human life, such as respiratory, cardiovascular, mental, and perinatal disorders ( 3 ), leading to infant mortality or chronic disease in adult age ( 6 ).
National reports have mentioned the increased risk of morbidity and mortality ( 1 ). These studies were conducted in many places around the world and show a correlation between daily ranges of particulate matter (PM) concentration and daily mortality. Climate shifts and global planetary warming ( 3 ) could aggravate the situation. Besides, increased hospitalization (an index of morbidity) has been registered among the elderly and susceptible individuals for specific reasons. Fine and ultrafine particulate matter seems to be associated with more serious illnesses ( 6 ), as it can invade the deepest parts of the airways and more easily reach the bloodstream.
Air pollution mainly affects those living in large urban areas, where road emissions contribute the most to the degradation of air quality. There is also a danger of industrial accidents, where the spread of a toxic fog can be fatal to the populations of the surrounding areas. The dispersion of pollutants is determined by many parameters, most notably atmospheric stability and wind ( 6 ).
In developing countries ( 7 ), the problem is more serious due to overpopulation and uncontrolled urbanization along with the development of industrialization. This leads to poor air quality, especially in countries with social disparities and a lack of information on sustainable management of the environment. The use of fuels such as wood fuel or solid fuel for domestic needs due to low incomes exposes people to bad-quality, polluted air at home. It is of note that three billion people around the world are using the above sources of energy for their daily heating and cooking needs ( 8 ). In developing countries, the women of the household seem to carry the highest risk for disease development due to their longer duration exposure to the indoor air pollution ( 8 , 9 ). Due to its fast industrial development and overpopulation, China is one of the Asian countries confronting serious air pollution problems ( 10 , 11 ). The lung cancer mortality observed in China is associated with fine particles ( 12 ). As stated already, long-term exposure is associated with deleterious effects on the cardiovascular system ( 3 , 5 ). However, it is interesting to note that cardiovascular diseases have mostly been observed in developed and high-income countries rather than in the developing low-income countries exposed highly to air pollution ( 13 ). Extreme air pollution is recorded in India, where the air quality reaches hazardous levels. New Delhi is one of the more polluted cities in India. Flights in and out of New Delhi International Airport are often canceled due to the reduced visibility associated with air pollution. Pollution is occurring both in urban and rural areas in India due to the fast industrialization, urbanization, and rise in use of motorcycle transportation. Nevertheless, biomass combustion associated with heating and cooking needs and practices is a major source of household air pollution in India and in Nepal ( 14 , 15 ). There is spatial heterogeneity in India, as areas with diverse climatological conditions and population and education levels generate different indoor air qualities, with higher PM 2.5 observed in North Indian states (557–601 μg/m 3 ) compared to the Southern States (183–214 μg/m 3 ) ( 16 , 17 ). The cold climate of the North Indian areas may be the main reason for this, as longer periods at home and more heating are necessary compared to in the tropical climate of Southern India. Household air pollution in India is associated with major health effects, especially in women and young children, who stay indoors for longer periods. Chronic obstructive respiratory disease (CORD) and lung cancer are mostly observed in women, while acute lower respiratory disease is seen in young children under 5 years of age ( 18 ).
Accumulation of air pollution, especially sulfur dioxide and smoke, reaching 1,500 mg/m3, resulted in an increase in the number of deaths (4,000 deaths) in December 1952 in London and in 1963 in New York City (400 deaths) ( 19 ). An association of pollution with mortality was reported on the basis of monitoring of outdoor pollution in six US metropolitan cities ( 20 ). In every case, it seems that mortality was closely related to the levels of fine, inhalable, and sulfate particles more than with the levels of total particulate pollution, aerosol acidity, sulfur dioxide, or nitrogen dioxide ( 20 ).
Furthermore, extremely high levels of pollution are reported in Mexico City and Rio de Janeiro, followed by Milan, Ankara, Melbourne, Tokyo, and Moscow ( 19 ).
Based on the magnitude of the public health impact, it is certain that different kinds of interventions should be taken into account. Success and effectiveness in controlling air pollution, specifically at the local level, have been reported. Adequate technological means are applied considering the source and the nature of the emission as well as its impact on health and the environment. The importance of point sources and non-point sources of air pollution control is reported by Schwela and Köth-Jahr ( 21 ). Without a doubt, a detailed emission inventory must record all sources in a given area. Beyond considering the above sources and their nature, topography and meteorology should also be considered, as stated previously. Assessment of the control policies and methods is often extrapolated from the local to the regional and then to the global scale. Air pollution may be dispersed and transported from one region to another area located far away. Air pollution management means the reduction to acceptable levels or possible elimination of air pollutants whose presence in the air affects our health or the environmental ecosystem. Private and governmental entities and authorities implement actions to ensure the air quality ( 22 ). Air quality standards and guidelines were adopted for the different pollutants by the WHO and EPA as a tool for the management of air quality ( 1 , 23 ). These standards have to be compared to the emissions inventory standards by causal analysis and dispersion modeling in order to reveal the problematic areas ( 24 ). Inventories are generally based on a combination of direct measurements and emissions modeling ( 24 ).
As an example, we state here the control measures at the source through the use of catalytic converters in cars. These are devices that turn the pollutants and toxic gases produced from combustion engines into less-toxic pollutants by catalysis through redox reactions ( 25 ). In Greece, the use of private cars was restricted by tracking their license plates in order to reduce traffic congestion during rush hour ( 25 ).
Concerning industrial emissions, collectors and closed systems can keep the air pollution to the minimal standards imposed by legislation ( 26 ).
Current strategies to improve air quality require an estimation of the economic value of the benefits gained from proposed programs. These proposed programs by public authorities, and directives are issued with guidelines to be respected.
In Europe, air quality limit values AQLVs (Air Quality Limit Values) are issued for setting off planning claims ( 27 ). In the USA, the NAAQS (National Ambient Air Quality Standards) establish the national air quality limit values ( 27 ). While both standards and directives are based on different mechanisms, significant success has been achieved in the reduction of overall emissions and associated health and environmental effects ( 27 ). The European Directive identifies geographical areas of risk exposure as monitoring/assessment zones to record the emission sources and levels of air pollution ( 27 ), whereas the USA establishes global geographical air quality criteria according to the severity of their air quality problem and records all sources of the pollutants and their precursors ( 27 ).
In this vein, funds have been financing, directly or indirectly, projects related to air quality along with the technical infrastructure to maintain good air quality. These plans focus on an inventory of databases from air quality environmental planning awareness campaigns. Moreover, pollution measures of air emissions may be taken for vehicles, machines, and industries in urban areas.
Technological innovation can only be successful if it is able to meet the needs of society. In this sense, technology must reflect the decision-making practices and procedures of those involved in risk assessment and evaluation and act as a facilitator in providing information and assessments to enable decision makers to make the best decisions possible. Summarizing the aforementioned in order to design an effective air quality control strategy, several aspects must be considered: environmental factors and ambient air quality conditions, engineering factors and air pollutant characteristics, and finally, economic operating costs for technological improvement and administrative and legal costs. Considering the economic factor, competitiveness through neoliberal concepts is offering a solution to environmental problems ( 22 ).
The development of environmental governance, along with technological progress, has initiated the deployment of a dialogue. Environmental politics has created objections and points of opposition between different political parties, scientists, media, and governmental and non-governmental organizations ( 22 ). Radical environmental activism actions and movements have been created ( 22 ). The rise of the new information and communication technologies (ICTs) are many times examined as to whether and in which way they have influenced means of communication and social movements such as activism ( 28 ). Since the 1990s, the term “digital activism” has been used increasingly and in many different disciplines ( 29 ). Nowadays, multiple digital technologies can be used to produce a digital activism outcome on environmental issues. More specifically, devices with online capabilities such as computers or mobile phones are being used as a way to pursue change in political and social affairs ( 30 ).
In the present paper, we focus on the sources of environmental pollution in relation to public health and propose some solutions and interventions that may be of interest to environmental legislators and decision makers.
Sources of Exposure
It is known that the majority of environmental pollutants are emitted through large-scale human activities such as the use of industrial machinery, power-producing stations, combustion engines, and cars. Because these activities are performed at such a large scale, they are by far the major contributors to air pollution, with cars estimated to be responsible for approximately 80% of today's pollution ( 31 ). Some other human activities are also influencing our environment to a lesser extent, such as field cultivation techniques, gas stations, fuel tanks heaters, and cleaning procedures ( 32 ), as well as several natural sources, such as volcanic and soil eruptions and forest fires.
The classification of air pollutants is based mainly on the sources producing pollution. Therefore, it is worth mentioning the four main sources, following the classification system: Major sources, Area sources, Mobile sources, and Natural sources.
Major sources include the emission of pollutants from power stations, refineries, and petrochemicals, the chemical and fertilizer industries, metallurgical and other industrial plants, and, finally, municipal incineration.
Indoor area sources include domestic cleaning activities, dry cleaners, printing shops, and petrol stations.
Mobile sources include automobiles, cars, railways, airways, and other types of vehicles.
Finally, natural sources include, as stated previously, physical disasters ( 33 ) such as forest fire, volcanic erosion, dust storms, and agricultural burning.
However, many classification systems have been proposed. Another type of classification is a grouping according to the recipient of the pollution, as follows:
Air pollution is determined as the presence of pollutants in the air in large quantities for long periods. Air pollutants are dispersed particles, hydrocarbons, CO, CO 2 , NO, NO 2 , SO 3 , etc.
Water pollution is organic and inorganic charge and biological charge ( 10 ) at high levels that affect the water quality ( 34 , 35 ).
Soil pollution occurs through the release of chemicals or the disposal of wastes, such as heavy metals, hydrocarbons, and pesticides.
Air pollution can influence the quality of soil and water bodies by polluting precipitation, falling into water and soil environments ( 34 , 36 ). Notably, the chemistry of the soil can be amended due to acid precipitation by affecting plants, cultures, and water quality ( 37 ). Moreover, movement of heavy metals is favored by soil acidity, and metals are so then moving into the watery environment. It is known that heavy metals such as aluminum are noxious to wildlife and fishes. Soil quality seems to be of importance, as soils with low calcium carbonate levels are at increased jeopardy from acid rain. Over and above rain, snow and particulate matter drip into watery ' bodies ( 36 , 38 ).
Lastly, pollution is classified following type of origin:
Radioactive and nuclear pollution , releasing radioactive and nuclear pollutants into water, air, and soil during nuclear explosions and accidents, from nuclear weapons, and through handling or disposal of radioactive sewage.
Radioactive materials can contaminate surface water bodies and, being noxious to the environment, plants, animals, and humans. It is known that several radioactive substances such as radium and uranium concentrate in the bones and can cause cancers ( 38 , 39 ).
Noise pollution is produced by machines, vehicles, traffic noises, and musical installations that are harmful to our hearing.
The World Health Organization introduced the term DALYs. The DALYs for a disease or health condition is defined as the sum of the Years of Life Lost (YLL) due to premature mortality in the population and the Years Lost due to Disability (YLD) for people living with the health condition or its consequences ( 39 ). In Europe, air pollution is the main cause of disability-adjusted life years lost (DALYs), followed by noise pollution. The potential relationships of noise and air pollution with health have been studied ( 40 ). The study found that DALYs related to noise were more important than those related to air pollution, as the effects of environmental noise on cardiovascular disease were independent of air pollution ( 40 ). Environmental noise should be counted as an independent public health risk ( 40 ).
Environmental pollution occurs when changes in the physical, chemical, or biological constituents of the environment (air masses, temperature, climate, etc.) are produced.
Pollutants harm our environment either by increasing levels above normal or by introducing harmful toxic substances. Primary pollutants are directly produced from the above sources, and secondary pollutants are emitted as by-products of the primary ones. Pollutants can be biodegradable or non-biodegradable and of natural origin or anthropogenic, as stated previously. Moreover, their origin can be a unique source (point-source) or dispersed sources.
Pollutants have differences in physical and chemical properties, explaining the discrepancy in their capacity for producing toxic effects. As an example, we state here that aerosol compounds ( 41 – 43 ) have a greater toxicity than gaseous compounds due to their tiny size (solid or liquid) in the atmosphere; they have a greater penetration capacity. Gaseous compounds are eliminated more easily by our respiratory system ( 41 ). These particles are able to damage lungs and can even enter the bloodstream ( 41 ), leading to the premature deaths of millions of people yearly. Moreover, the aerosol acidity ([H+]) seems to considerably enhance the production of secondary organic aerosols (SOA), but this last aspect is not supported by other scientific teams ( 38 ).
Climate and Pollution
Air pollution and climate change are closely related. Climate is the other side of the same coin that reduces the quality of our Earth ( 44 ). Pollutants such as black carbon, methane, tropospheric ozone, and aerosols affect the amount of incoming sunlight. As a result, the temperature of the Earth is increasing, resulting in the melting of ice, icebergs, and glaciers.
In this vein, climatic changes will affect the incidence and prevalence of both residual and imported infections in Europe. Climate and weather affect the duration, timing, and intensity of outbreaks strongly and change the map of infectious diseases in the globe ( 45 ). Mosquito-transmitted parasitic or viral diseases are extremely climate-sensitive, as warming firstly shortens the pathogen incubation period and secondly shifts the geographic map of the vector. Similarly, water-warming following climate changes leads to a high incidence of waterborne infections. Recently, in Europe, eradicated diseases seem to be emerging due to the migration of population, for example, cholera, poliomyelitis, tick-borne encephalitis, and malaria ( 46 ).
The spread of epidemics is associated with natural climate disasters and storms, which seem to occur more frequently nowadays ( 47 ). Malnutrition and disequilibration of the immune system are also associated with the emerging infections affecting public health ( 48 ).
The Chikungunya virus “took the airplane” from the Indian Ocean to Europe, as outbreaks of the disease were registered in Italy ( 49 ) as well as autochthonous cases in France ( 50 ).
An increase in cryptosporidiosis in the United Kingdom and in the Czech Republic seems to have occurred following flooding ( 36 , 51 ).
As stated previously, aerosols compounds are tiny in size and considerably affect the climate. They are able to dissipate sunlight (the albedo phenomenon) by dispersing a quarter of the sun's rays back to space and have cooled the global temperature over the last 30 years ( 52 ).
Air Pollutants
The World Health Organization (WHO) reports on six major air pollutants, namely particle pollution, ground-level ozone, carbon monoxide, sulfur oxides, nitrogen oxides, and lead. Air pollution can have a disastrous effect on all components of the environment, including groundwater, soil, and air. Additionally, it poses a serious threat to living organisms. In this vein, our interest is mainly to focus on these pollutants, as they are related to more extensive and severe problems in human health and environmental impact. Acid rain, global warming, the greenhouse effect, and climate changes have an important ecological impact on air pollution ( 53 ).
Particulate Matter (PM) and Health
Studies have shown a relationship between particulate matter (PM) and adverse health effects, focusing on either short-term (acute) or long-term (chronic) PM exposure.
Particulate matter (PM) is usually formed in the atmosphere as a result of chemical reactions between the different pollutants. The penetration of particles is closely dependent on their size ( 53 ). Particulate Matter (PM) was defined as a term for particles by the United States Environmental Protection Agency ( 54 ). Particulate matter (PM) pollution includes particles with diameters of 10 micrometers (μm) or smaller, called PM 10 , and extremely fine particles with diameters that are generally 2.5 micrometers (μm) and smaller.
Particulate matter contains tiny liquid or solid droplets that can be inhaled and cause serious health effects ( 55 ). Particles <10 μm in diameter (PM 10 ) after inhalation can invade the lungs and even reach the bloodstream. Fine particles, PM 2.5 , pose a greater risk to health ( 6 , 56 ) ( Table 1 ).
Penetrability according to particle size.
Multiple epidemiological studies have been performed on the health effects of PM. A positive relation was shown between both short-term and long-term exposures of PM 2.5 and acute nasopharyngitis ( 56 ). In addition, long-term exposure to PM for years was found to be related to cardiovascular diseases and infant mortality.
Those studies depend on PM 2.5 monitors and are restricted in terms of study area or city area due to a lack of spatially resolved daily PM 2.5 concentration data and, in this way, are not representative of the entire population. Following a recent epidemiological study by the Department of Environmental Health at Harvard School of Public Health (Boston, MA) ( 57 ), it was reported that, as PM 2.5 concentrations vary spatially, an exposure error (Berkson error) seems to be produced, and the relative magnitudes of the short- and long-term effects are not yet completely elucidated. The team developed a PM 2.5 exposure model based on remote sensing data for assessing short- and long-term human exposures ( 57 ). This model permits spatial resolution in short-term effects plus the assessment of long-term effects in the whole population.
Moreover, respiratory diseases and affection of the immune system are registered as long-term chronic effects ( 58 ). It is worth noting that people with asthma, pneumonia, diabetes, and respiratory and cardiovascular diseases are especially susceptible and vulnerable to the effects of PM. PM 2.5 , followed by PM 10 , are strongly associated with diverse respiratory system diseases ( 59 ), as their size permits them to pierce interior spaces ( 60 ). The particles produce toxic effects according to their chemical and physical properties. The components of PM 10 and PM 2.5 can be organic (polycyclic aromatic hydrocarbons, dioxins, benzene, 1-3 butadiene) or inorganic (carbon, chlorides, nitrates, sulfates, metals) in nature ( 55 ).
Particulate Matter (PM) is divided into four main categories according to type and size ( 61 ) ( Table 2 ).
Types and sizes of particulate Matter (PM).
Gas contaminants include PM in aerial masses.
Particulate contaminants include contaminants such as smog, soot, tobacco smoke, oil smoke, fly ash, and cement dust.
Biological Contaminants are microorganisms (bacteria, viruses, fungi, mold, and bacterial spores), cat allergens, house dust and allergens, and pollen.
Types of Dust include suspended atmospheric dust, settling dust, and heavy dust.
Finally, another fact is that the half-lives of PM 10 and PM 2.5 particles in the atmosphere is extended due to their tiny dimensions; this permits their long-lasting suspension in the atmosphere and even their transfer and spread to distant destinations where people and the environment may be exposed to the same magnitude of pollution ( 53 ). They are able to change the nutrient balance in watery ecosystems, damage forests and crops, and acidify water bodies.
As stated, PM 2.5 , due to their tiny size, are causing more serious health effects. These aforementioned fine particles are the main cause of the “haze” formation in different metropolitan areas ( 12 , 13 , 61 ).
Ozone Impact in the Atmosphere
Ozone (O 3 ) is a gas formed from oxygen under high voltage electric discharge ( 62 ). It is a strong oxidant, 52% stronger than chlorine. It arises in the stratosphere, but it could also arise following chain reactions of photochemical smog in the troposphere ( 63 ).
Ozone can travel to distant areas from its initial source, moving with air masses ( 64 ). It is surprising that ozone levels over cities are low in contrast to the increased amounts occuring in urban areas, which could become harmful for cultures, forests, and vegetation ( 65 ) as it is reducing carbon assimilation ( 66 ). Ozone reduces growth and yield ( 47 , 48 ) and affects the plant microflora due to its antimicrobial capacity ( 67 , 68 ). In this regard, ozone acts upon other natural ecosystems, with microflora ( 69 , 70 ) and animal species changing their species composition ( 71 ). Ozone increases DNA damage in epidermal keratinocytes and leads to impaired cellular function ( 72 ).
Ground-level ozone (GLO) is generated through a chemical reaction between oxides of nitrogen and VOCs emitted from natural sources and/or following anthropogenic activities.
Ozone uptake usually occurs by inhalation. Ozone affects the upper layers of the skin and the tear ducts ( 73 ). A study of short-term exposure of mice to high levels of ozone showed malondialdehyde formation in the upper skin (epidermis) but also depletion in vitamins C and E. It is likely that ozone levels are not interfering with the skin barrier function and integrity to predispose to skin disease ( 74 ).
Due to the low water-solubility of ozone, inhaled ozone has the capacity to penetrate deeply into the lungs ( 75 ).
Toxic effects induced by ozone are registered in urban areas all over the world, causing biochemical, morphologic, functional, and immunological disorders ( 76 ).
The European project (APHEA2) focuses on the acute effects of ambient ozone concentrations on mortality ( 77 ). Daily ozone concentrations compared to the daily number of deaths were reported from different European cities for a 3-year period. During the warm period of the year, an observed increase in ozone concentration was associated with an increase in the daily number of deaths (0.33%), in the number of respiratory deaths (1.13%), and in the number of cardiovascular deaths (0.45%). No effect was observed during wintertime.
Carbon Monoxide (CO)
Carbon monoxide is produced by fossil fuel when combustion is incomplete. The symptoms of poisoning due to inhaling carbon monoxide include headache, dizziness, weakness, nausea, vomiting, and, finally, loss of consciousness.
The affinity of carbon monoxide to hemoglobin is much greater than that of oxygen. In this vein, serious poisoning may occur in people exposed to high levels of carbon monoxide for a long period of time. Due to the loss of oxygen as a result of the competitive binding of carbon monoxide, hypoxia, ischemia, and cardiovascular disease are observed.
Carbon monoxide affects the greenhouses gases that are tightly connected to global warming and climate. This should lead to an increase in soil and water temperatures, and extreme weather conditions or storms may occur ( 68 ).
However, in laboratory and field experiments, it has been seen to produce increased plant growth ( 78 ).
Nitrogen Oxide (NO 2 )
Nitrogen oxide is a traffic-related pollutant, as it is emitted from automobile motor engines ( 79 , 80 ). It is an irritant of the respiratory system as it penetrates deep in the lung, inducing respiratory diseases, coughing, wheezing, dyspnea, bronchospasm, and even pulmonary edema when inhaled at high levels. It seems that concentrations over 0.2 ppm produce these adverse effects in humans, while concentrations higher than 2.0 ppm affect T-lymphocytes, particularly the CD8+ cells and NK cells that produce our immune response ( 81 ).It is reported that long-term exposure to high levels of nitrogen dioxide can be responsible for chronic lung disease. Long-term exposure to NO 2 can impair the sense of smell ( 81 ).
However, systems other than respiratory ones can be involved, as symptoms such as eye, throat, and nose irritation have been registered ( 81 ).
High levels of nitrogen dioxide are deleterious to crops and vegetation, as they have been observed to reduce crop yield and plant growth efficiency. Moreover, NO 2 can reduce visibility and discolor fabrics ( 81 ).
Sulfur Dioxide (SO 2 )
Sulfur dioxide is a harmful gas that is emitted mainly from fossil fuel consumption or industrial activities. The annual standard for SO 2 is 0.03 ppm ( 82 ). It affects human, animal, and plant life. Susceptible people as those with lung disease, old people, and children, who present a higher risk of damage. The major health problems associated with sulfur dioxide emissions in industrialized areas are respiratory irritation, bronchitis, mucus production, and bronchospasm, as it is a sensory irritant and penetrates deep into the lung converted into bisulfite and interacting with sensory receptors, causing bronchoconstriction. Moreover, skin redness, damage to the eyes (lacrimation and corneal opacity) and mucous membranes, and worsening of pre-existing cardiovascular disease have been observed ( 81 ).
Environmental adverse effects, such as acidification of soil and acid rain, seem to be associated with sulfur dioxide emissions ( 83 ).
Lead is a heavy metal used in different industrial plants and emitted from some petrol motor engines, batteries, radiators, waste incinerators, and waste waters ( 84 ).
Moreover, major sources of lead pollution in the air are metals, ore, and piston-engine aircraft. Lead poisoning is a threat to public health due to its deleterious effects upon humans, animals, and the environment, especially in the developing countries.
Exposure to lead can occur through inhalation, ingestion, and dermal absorption. Trans- placental transport of lead was also reported, as lead passes through the placenta unencumbered ( 85 ). The younger the fetus is, the more harmful the toxic effects. Lead toxicity affects the fetal nervous system; edema or swelling of the brain is observed ( 86 ). Lead, when inhaled, accumulates in the blood, soft tissue, liver, lung, bones, and cardiovascular, nervous, and reproductive systems. Moreover, loss of concentration and memory, as well as muscle and joint pain, were observed in adults ( 85 , 86 ).
Children and newborns ( 87 ) are extremely susceptible even to minimal doses of lead, as it is a neurotoxicant and causes learning disabilities, impairment of memory, hyperactivity, and even mental retardation.
Elevated amounts of lead in the environment are harmful to plants and crop growth. Neurological effects are observed in vertebrates and animals in association with high lead levels ( 88 ).
Polycyclic Aromatic Hydrocarbons(PAHs)
The distribution of PAHs is ubiquitous in the environment, as the atmosphere is the most important means of their dispersal. They are found in coal and in tar sediments. Moreover, they are generated through incomplete combustion of organic matter as in the cases of forest fires, incineration, and engines ( 89 ). PAH compounds, such as benzopyrene, acenaphthylene, anthracene, and fluoranthene are recognized as toxic, mutagenic, and carcinogenic substances. They are an important risk factor for lung cancer ( 89 ).
Volatile Organic Compounds(VOCs)
Volatile organic compounds (VOCs), such as toluene, benzene, ethylbenzene, and xylene ( 90 ), have been found to be associated with cancer in humans ( 91 ). The use of new products and materials has actually resulted in increased concentrations of VOCs. VOCs pollute indoor air ( 90 ) and may have adverse effects on human health ( 91 ). Short-term and long-term adverse effects on human health are observed. VOCs are responsible for indoor air smells. Short-term exposure is found to cause irritation of eyes, nose, throat, and mucosal membranes, while those of long duration exposure include toxic reactions ( 92 ). Predictable assessment of the toxic effects of complex VOC mixtures is difficult to estimate, as these pollutants can have synergic, antagonistic, or indifferent effects ( 91 , 93 ).
Dioxins originate from industrial processes but also come from natural processes, such as forest fires and volcanic eruptions. They accumulate in foods such as meat and dairy products, fish and shellfish, and especially in the fatty tissue of animals ( 94 ).
Short-period exhibition to high dioxin concentrations may result in dark spots and lesions on the skin ( 94 ). Long-term exposure to dioxins can cause developmental problems, impairment of the immune, endocrine and nervous systems, reproductive infertility, and cancer ( 94 ).
Without any doubt, fossil fuel consumption is responsible for a sizeable part of air contamination. This contamination may be anthropogenic, as in agricultural and industrial processes or transportation, while contamination from natural sources is also possible. Interestingly, it is of note that the air quality standards established through the European Air Quality Directive are somewhat looser than the WHO guidelines, which are stricter ( 95 ).
Effect of Air Pollution on Health
The most common air pollutants are ground-level ozone and Particulates Matter (PM). Air pollution is distinguished into two main types:
Outdoor pollution is the ambient air pollution.
Indoor pollution is the pollution generated by household combustion of fuels.
People exposed to high concentrations of air pollutants experience disease symptoms and states of greater and lesser seriousness. These effects are grouped into short- and long-term effects affecting health.
Susceptible populations that need to be aware of health protection measures include old people, children, and people with diabetes and predisposing heart or lung disease, especially asthma.
As extensively stated previously, according to a recent epidemiological study from Harvard School of Public Health, the relative magnitudes of the short- and long-term effects have not been completely clarified ( 57 ) due to the different epidemiological methodologies and to the exposure errors. New models are proposed for assessing short- and long-term human exposure data more successfully ( 57 ). Thus, in the present section, we report the more common short- and long-term health effects but also general concerns for both types of effects, as these effects are often dependent on environmental conditions, dose, and individual susceptibility.
Short-term effects are temporary and range from simple discomfort, such as irritation of the eyes, nose, skin, throat, wheezing, coughing and chest tightness, and breathing difficulties, to more serious states, such as asthma, pneumonia, bronchitis, and lung and heart problems. Short-term exposure to air pollution can also cause headaches, nausea, and dizziness.
These problems can be aggravated by extended long-term exposure to the pollutants, which is harmful to the neurological, reproductive, and respiratory systems and causes cancer and even, rarely, deaths.
The long-term effects are chronic, lasting for years or the whole life and can even lead to death. Furthermore, the toxicity of several air pollutants may also induce a variety of cancers in the long term ( 96 ).
As stated already, respiratory disorders are closely associated with the inhalation of air pollutants. These pollutants will invade through the airways and will accumulate at the cells. Damage to target cells should be related to the pollutant component involved and its source and dose. Health effects are also closely dependent on country, area, season, and time. An extended exposure duration to the pollutant should incline to long-term health effects in relation also to the above factors.
Particulate Matter (PMs), dust, benzene, and O 3 cause serious damage to the respiratory system ( 97 ). Moreover, there is a supplementary risk in case of existing respiratory disease such as asthma ( 98 ). Long-term effects are more frequent in people with a predisposing disease state. When the trachea is contaminated by pollutants, voice alterations may be remarked after acute exposure. Chronic obstructive pulmonary disease (COPD) may be induced following air pollution, increasing morbidity and mortality ( 99 ). Long-term effects from traffic, industrial air pollution, and combustion of fuels are the major factors for COPD risk ( 99 ).
Multiple cardiovascular effects have been observed after exposure to air pollutants ( 100 ). Changes occurred in blood cells after long-term exposure may affect cardiac functionality. Coronary arteriosclerosis was reported following long-term exposure to traffic emissions ( 101 ), while short-term exposure is related to hypertension, stroke, myocardial infracts, and heart insufficiency. Ventricle hypertrophy is reported to occur in humans after long-time exposure to nitrogen oxide (NO 2 ) ( 102 , 103 ).
Neurological effects have been observed in adults and children after extended-term exposure to air pollutants.
Psychological complications, autism, retinopathy, fetal growth, and low birth weight seem to be related to long-term air pollution ( 83 ). The etiologic agent of the neurodegenerative diseases (Alzheimer's and Parkinson's) is not yet known, although it is believed that extended exposure to air pollution seems to be a factor. Specifically, pesticides and metals are cited as etiological factors, together with diet. The mechanisms in the development of neurodegenerative disease include oxidative stress, protein aggregation, inflammation, and mitochondrial impairment in neurons ( 104 ) ( Figure 1 ).
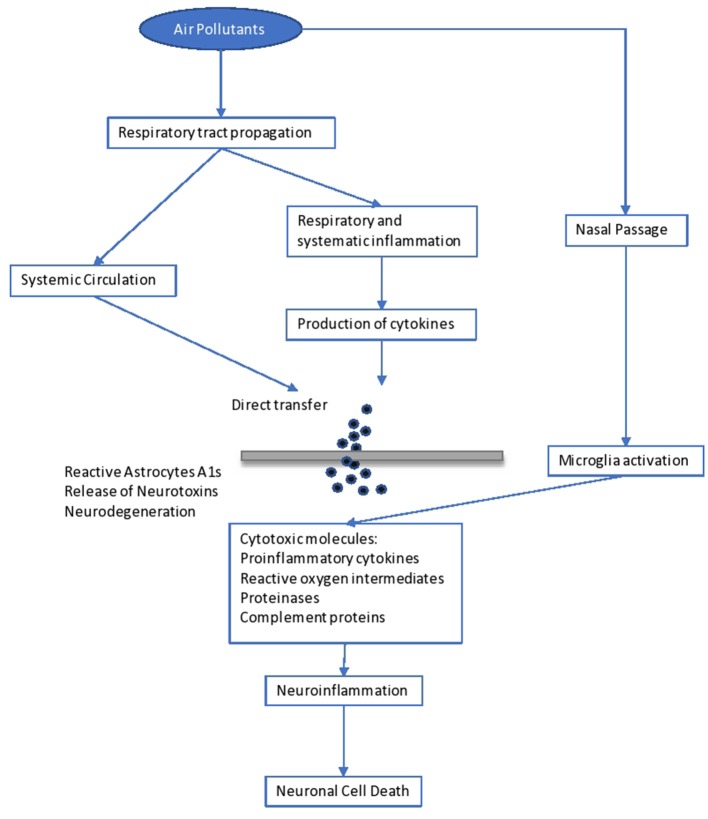
Impact of air pollutants on the brain.
Brain inflammation was observed in dogs living in a highly polluted area in Mexico for a long period ( 105 ). In human adults, markers of systemic inflammation (IL-6 and fibrinogen) were found to be increased as an immediate response to PNC on the IL-6 level, possibly leading to the production of acute-phase proteins ( 106 ). The progression of atherosclerosis and oxidative stress seem to be the mechanisms involved in the neurological disturbances caused by long-term air pollution. Inflammation comes secondary to the oxidative stress and seems to be involved in the impairment of developmental maturation, affecting multiple organs ( 105 , 107 ). Similarly, other factors seem to be involved in the developmental maturation, which define the vulnerability to long-term air pollution. These include birthweight, maternal smoking, genetic background and socioeconomic environment, as well as education level.
However, diet, starting from breast-feeding, is another determinant factor. Diet is the main source of antioxidants, which play a key role in our protection against air pollutants ( 108 ). Antioxidants are free radical scavengers and limit the interaction of free radicals in the brain ( 108 ). Similarly, genetic background may result in a differential susceptibility toward the oxidative stress pathway ( 60 ). For example, antioxidant supplementation with vitamins C and E appears to modulate the effect of ozone in asthmatic children homozygous for the GSTM1 null allele ( 61 ). Inflammatory cytokines released in the periphery (e.g., respiratory epithelia) upregulate the innate immune Toll-like receptor 2. Such activation and the subsequent events leading to neurodegeneration have recently been observed in lung lavage in mice exposed to ambient Los Angeles (CA, USA) particulate matter ( 61 ). In children, neurodevelopmental morbidities were observed after lead exposure. These children developed aggressive and delinquent behavior, reduced intelligence, learning difficulties, and hyperactivity ( 109 ). No level of lead exposure seems to be “safe,” and the scientific community has asked the Centers for Disease Control and Prevention (CDC) to reduce the current screening guideline of 10 μg/dl ( 109 ).
It is important to state that impact on the immune system, causing dysfunction and neuroinflammation ( 104 ), is related to poor air quality. Yet, increases in serum levels of immunoglobulins (IgA, IgM) and the complement component C3 are observed ( 106 ). Another issue is that antigen presentation is affected by air pollutants, as there is an upregulation of costimulatory molecules such as CD80 and CD86 on macrophages ( 110 ).
As is known, skin is our shield against ultraviolet radiation (UVR) and other pollutants, as it is the most exterior layer of our body. Traffic-related pollutants, such as PAHs, VOCs, oxides, and PM, may cause pigmented spots on our skin ( 111 ). On the one hand, as already stated, when pollutants penetrate through the skin or are inhaled, damage to the organs is observed, as some of these pollutants are mutagenic and carcinogenic, and, specifically, they affect the liver and lung. On the other hand, air pollutants (and those in the troposphere) reduce the adverse effects of ultraviolet radiation UVR in polluted urban areas ( 111 ). Air pollutants absorbed by the human skin may contribute to skin aging, psoriasis, acne, urticaria, eczema, and atopic dermatitis ( 111 ), usually caused by exposure to oxides and photochemical smoke ( 111 ). Exposure to PM and cigarette smoking act as skin-aging agents, causing spots, dyschromia, and wrinkles. Lastly, pollutants have been associated with skin cancer ( 111 ).
Higher morbidity is reported to fetuses and children when exposed to the above dangers. Impairment in fetal growth, low birth weight, and autism have been reported ( 112 ).
Another exterior organ that may be affected is the eye. Contamination usually comes from suspended pollutants and may result in asymptomatic eye outcomes, irritation ( 112 ), retinopathy, or dry eye syndrome ( 113 , 114 ).
Environmental Impact of Air Pollution
Air pollution is harming not only human health but also the environment ( 115 ) in which we live. The most important environmental effects are as follows.
Acid rain is wet (rain, fog, snow) or dry (particulates and gas) precipitation containing toxic amounts of nitric and sulfuric acids. They are able to acidify the water and soil environments, damage trees and plantations, and even damage buildings and outdoor sculptures, constructions, and statues.
Haze is produced when fine particles are dispersed in the air and reduce the transparency of the atmosphere. It is caused by gas emissions in the air coming from industrial facilities, power plants, automobiles, and trucks.
Ozone , as discussed previously, occurs both at ground level and in the upper level (stratosphere) of the Earth's atmosphere. Stratospheric ozone is protecting us from the Sun's harmful ultraviolet (UV) rays. In contrast, ground-level ozone is harmful to human health and is a pollutant. Unfortunately, stratospheric ozone is gradually damaged by ozone-depleting substances (i.e., chemicals, pesticides, and aerosols). If this protecting stratospheric ozone layer is thinned, then UV radiation can reach our Earth, with harmful effects for human life (skin cancer) ( 116 ) and crops ( 117 ). In plants, ozone penetrates through the stomata, inducing them to close, which blocks CO 2 transfer and induces a reduction in photosynthesis ( 118 ).
Global climate change is an important issue that concerns mankind. As is known, the “greenhouse effect” keeps the Earth's temperature stable. Unhappily, anthropogenic activities have destroyed this protecting temperature effect by producing large amounts of greenhouse gases, and global warming is mounting, with harmful effects on human health, animals, forests, wildlife, agriculture, and the water environment. A report states that global warming is adding to the health risks of poor people ( 119 ).
People living in poorly constructed buildings in warm-climate countries are at high risk for heat-related health problems as temperatures mount ( 119 ).
Wildlife is burdened by toxic pollutants coming from the air, soil, or the water ecosystem and, in this way, animals can develop health problems when exposed to high levels of pollutants. Reproductive failure and birth effects have been reported.
Eutrophication is occurring when elevated concentrations of nutrients (especially nitrogen) stimulate the blooming of aquatic algae, which can cause a disequilibration in the diversity of fish and their deaths.
Without a doubt, there is a critical concentration of pollution that an ecosystem can tolerate without being destroyed, which is associated with the ecosystem's capacity to neutralize acidity. The Canada Acid Rain Program established this load at 20 kg/ha/yr ( 120 ).
Hence, air pollution has deleterious effects on both soil and water ( 121 ). Concerning PM as an air pollutant, its impact on crop yield and food productivity has been reported. Its impact on watery bodies is associated with the survival of living organisms and fishes and their productivity potential ( 121 ).
An impairment in photosynthetic rhythm and metabolism is observed in plants exposed to the effects of ozone ( 121 ).
Sulfur and nitrogen oxides are involved in the formation of acid rain and are harmful to plants and marine organisms.
Last but not least, as mentioned above, the toxicity associated with lead and other metals is the main threat to our ecosystems (air, water, and soil) and living creatures ( 121 ).
In 2018, during the first WHO Global Conference on Air Pollution and Health, the WHO's General Director, Dr. Tedros Adhanom Ghebreyesus, called air pollution a “silent public health emergency” and “the new tobacco” ( 122 ).
Undoubtedly, children are particularly vulnerable to air pollution, especially during their development. Air pollution has adverse effects on our lives in many different respects.
Diseases associated with air pollution have not only an important economic impact but also a societal impact due to absences from productive work and school.
Despite the difficulty of eradicating the problem of anthropogenic environmental pollution, a successful solution could be envisaged as a tight collaboration of authorities, bodies, and doctors to regularize the situation. Governments should spread sufficient information and educate people and should involve professionals in these issues so as to control the emergence of the problem successfully.
Technologies to reduce air pollution at the source must be established and should be used in all industries and power plants. The Kyoto Protocol of 1997 set as a major target the reduction of GHG emissions to below 5% by 2012 ( 123 ). This was followed by the Copenhagen summit, 2009 ( 124 ), and then the Durban summit of 2011 ( 125 ), where it was decided to keep to the same line of action. The Kyoto protocol and the subsequent ones were ratified by many countries. Among the pioneers who adopted this important protocol for the world's environmental and climate “health” was China ( 3 ). As is known, China is a fast-developing economy and its GDP (Gross Domestic Product) is expected to be very high by 2050, which is defined as the year of dissolution of the protocol for the decrease in gas emissions.
A more recent international agreement of crucial importance for climate change is the Paris Agreement of 2015, issued by the UNFCCC (United Nations Climate Change Committee). This latest agreement was ratified by a plethora of UN (United Nations) countries as well as the countries of the European Union ( 126 ). In this vein, parties should promote actions and measures to enhance numerous aspects around the subject. Boosting education, training, public awareness, and public participation are some of the relevant actions for maximizing the opportunities to achieve the targets and goals on the crucial matter of climate change and environmental pollution ( 126 ). Without any doubt, technological improvements makes our world easier and it seems difficult to reduce the harmful impact caused by gas emissions, we could limit its use by seeking reliable approaches.
Synopsizing, a global prevention policy should be designed in order to combat anthropogenic air pollution as a complement to the correct handling of the adverse health effects associated with air pollution. Sustainable development practices should be applied, together with information coming from research in order to handle the problem effectively.
At this point, international cooperation in terms of research, development, administration policy, monitoring, and politics is vital for effective pollution control. Legislation concerning air pollution must be aligned and updated, and policy makers should propose the design of a powerful tool of environmental and health protection. As a result, the main proposal of this essay is that we should focus on fostering local structures to promote experience and practice and extrapolate these to the international level through developing effective policies for sustainable management of ecosystems.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
IM is employed by the company Delphis S.A. The remaining authors declare that the present review paper was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- 1. WHO Air Pollution. WHO. Available online at: http://www.who.int/airpollution/en/ (accessed October 5, 2019).
- 2. Moores FC. Climate change and air pollution: exploring the synergies and potential for mitigation in industrializing countries. Sustainability. (2009) 1:43–54. 10.3390/su1010043 [ DOI ] [ Google Scholar ]
- 3. USGCRP (2009). Global Climate Change Impacts in the United States. In: Karl TR, Melillo JM, Peterson TC, editors. Climate Change Impacts by Sectors: Ecosystems. New York, NY: United States Global Change Research Program. Cambridge University Press. [ Google Scholar ]
- 4. Marlon JR, Bloodhart B, Ballew MT, Rolfe-Redding J, Roser-Renouf C, Leiserowitz A, et al. (2019). How hope and doubt affect climate change mobilization. Front. Commun. 4:20 10.3389/fcomm.2019.00020 [ DOI ] [ Google Scholar ]
- 5. Eze IC, Schaffner E, Fischer E, Schikowski T, Adam M, Imboden M, et al. Long- term air pollution exposure and diabetes in a population-based Swiss cohort. Environ Int. (2014) 70:95–105. 10.1016/j.envint.2014.05.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Kelishadi R, Poursafa P. Air pollution and non-respiratory health hazards for children. Arch Med Sci. (2010) 6:483–95. 10.5114/aoms.2010.14458 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Manucci PM, Franchini M. Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health. (2017) 14:1048 10.3390/ijerph14091048 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Burden of Disease from Ambient and Household Air Pollution. Available online: http://who.int/phe/health_topics/outdoorair/databases/en/ (accessed August 15, 2017).
- 9. Hashim D, Boffetta P. Occupational and environmental exposures and cancers in developing countries. Ann Glob Health. (2014) 80:393–411. 10.1016/j.aogh.2014.10.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Guo Y, Zeng H, Zheng R, Li S, Pereira G, Liu Q, et al. The burden of lung cancer mortality attributable to fine particles in China. Total Environ Sci. (2017) 579:1460–6. 10.1016/j.scitotenv.2016.11.147 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Hou Q, An XQ, Wang Y, Guo JP. An evaluation of resident exposure to respirable particulate matter and health economic loss in Beijing during Beijing 2008 Olympic Games. Sci Total Environ. (2010) 408:4026–32. 10.1016/j.scitotenv.2009.12.030 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Kan H, Chen R, Tong S. Ambient air pollution, climate change, and population health in China. Environ Int. (2012) 42:10–9. 10.1016/j.envint.2011.03.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Burroughs Peña MS, Rollins A. Environmental exposures and cardiovascular disease: a challenge for health and development in low- and middle-income countries. Cardiol Clin. (2017) 35:71–86. 10.1016/j.ccl.2016.09.001 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Kankaria A, Nongkynrih B, Gupta S. Indoor air pollution in india: implications on health and its control. Indian J Comm Med. 39:203–7. 10.4103/0970-0218.143019 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Parajuli I, Lee H, Shrestha KR. Indoor air quality and ventilation assessment of rural mountainous households of Nepal. Int J Sust Built Env. (2016) 5:301–11. 10.1016/j.ijsbe.2016.08.003 [ DOI ] [ Google Scholar ]
- 16. Saud T, Gautam R, Mandal TK, Gadi R, Singh DP, Sharma SK. Emission estimates of organic and elemental carbon from household biomass fuel used over the Indo-Gangetic Plain (IGP), India. Atmos Environ. (2012) 61:212–20. 10.1016/j.atmosenv.2012.07.030 [ DOI ] [ Google Scholar ]
- 17. Singh DP, Gadi R, Mandal TK, Saud T, Saxena M, Sharma SK. Emissions estimates of PAH from biomass fuels used in rural sector of Indo-Gangetic Plains of India. Atmos Environ. (2013) 68:120–6. 10.1016/j.atmosenv.2012.11.042 [ DOI ] [ Google Scholar ]
- 18. Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M BN. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. (2008) 86:390–4. 10.2471/BLT.07.044529 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Kassomenos P, Kelessis A, Petrakakis M, Zoumakis N, Christides T, Paschalidou AK. Air Quality assessment in a heavily-polluted urban Mediterranean environment through Air Quality indices. Ecol Indic. (2012) 18:259–68. 10.1016/j.ecolind.2011.11.021 [ DOI ] [ Google Scholar ]
- 20. Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. (1993) 329:1753–9. 10.1056/NEJM199312093292401 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Schwela DH, Köth-Jahr I. Leitfaden für die Aufstellung von Luftreinhalteplänen [Guidelines for the Implementation of Clean Air Implementation Plans]. Landesumweltamt des Landes Nordrhein Westfalen. State Environmental Service of the State of North Rhine-Westphalia (1994). [ Google Scholar ]
- 22. Newlands M. Environmental Activism, Environmental Politics, and Representation: The Framing of the British Environmental Activist Movement. Ph.D. thesis. University of East London, United Kingdom (2015). [ Google Scholar ]
- 23. NEPIS (National Service Center for Environmental Publications) US EPA (Environmental Protection Agency) (2017). Available online at: https://www.epa.gov/clean-air-act-overview/air-pollution-current-and-future-challenges (accessed August 15, 2017).
- 24. NRC (National Research Council) Available online at: https://www.nap.edu/read/10728/chapter/1,2014 (accessed September 17, 2019).
- 25. Bull A. Traffic Congestion: The Problem and How to Deal With It. Santiago: Nationes Unidas, Cepal; (2003). [ Google Scholar ]
- 26. Spiegel J, Maystre LY. Environmental Pollution Control, Part VII - The Environment, Chapter 55, Encyclopedia of Occupational Health and Safety. Available online at: http://www.ilocis.org/documents/chpt55e.htm (accessed September 17, 2019).
- 27. European Community Reports Assessment of the Effectiveness of European Air Quality Policies and Measures: Case Study 2; Comparison of the EU and US Air Quality Standards and Planning Requirements. (2004). Available online at: https://ec.europa.eu/environment/archives/cafe/activities/pdf/case_study2.pdf (accessed September 22, 2019).
- 28. Gibson R, Ward S. Parties in the digital age; a review. J Represent Democracy. (2009) 45:87–100. 10.1080/00344890802710888 [ DOI ] [ Google Scholar ]
- 29. Kaun A, Uldam J. Digital activism: after the hype. New Media Soc. (2017) 20:2099–106. 10.1177/14614448177319 [ DOI ] [ Google Scholar ]
- 30. Sivitanides M, Shah V. The era of digital activism. In: 2011 Conference for Information Systems Applied Research(CONISAR) Proceedings Wilmington North Carolina, USA. Available online at: https://www.arifyildirim.com/ilt510/marcos.sivitanides.vivek.shah.pdf (accessed September 22, 2019).
- 31. Möller L, Schuetzle D, Autrup H. Future research needs associated with the assessment of potential human health risks from exposure to toxic ambient air pollutants. Environ Health Perspect. (1994) 102(Suppl. 4):193–210. 10.1289/ehp.94102s4193 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Jacobson MZ, Jacobson PMZ. Atmospheric Pollution: History, Science, and Regulation. Cambridge University Press (2002). p. 206 10.1256/wea.243.02 [ DOI ] [ Google Scholar ]
- 33. Stover RH. Flooding of soil for disease control. In: Mulder D, editor. Chapter 3. Developments in Agricultural and Managed Forest Ecology. Elsevier (1979). p. 19–28. Available online at: http://www.sciencedirect.com/science/article/pii/B9780444416926500094 10.1016/B978-0-444-41692-6.50009-4 (accessed July 1, 2019). [ DOI ]
- 34. Maipa V, Alamanos Y, Bezirtzoglou E. Seasonal fluctuation of bacterial indicators in coastal waters. Microb Ecol Health Dis. (2001) 13:143–6. 10.1080/089106001750462687 [ DOI ] [ Google Scholar ]
- 35. Bezirtzoglou E, Dimitriou D, Panagiou A. Occurrence of Clostridium perfringens in river water by using a new procedure. Anaerobe. (1996) 2:169–73. 10.1006/anae.1996.0022 [ DOI ] [ Google Scholar ]
- 36. Kjellstrom T, Lodh M, McMichael T, Ranmuthugala G, Shrestha R, Kingsland S. Air and Water Pollution: Burden and Strategies for Control. DCP, Chapter 43. 817–32 p. Available online at: https://www.dcp-3.org/sites/default/files/dcp2/DCP43.pdf (accessed September 17, 2017).
- 37. Pathak RK, Wang T, Ho KF, Lee SC. Characteristics of summertime PM2.5 organic and elemental carbon in four major Chinese cities: implications of high acidity for water- soluble organic carbon (WSOC). Atmos Environ. (2011) 45:318–25. 10.1016/j.atmosenv.2010.10.021 [ DOI ] [ Google Scholar ]
- 38. Bonavigo L, Zucchetti M, Mankolli H. Water radioactive pollution and related environmental aspects. J Int Env Appl Sci. (2009) 4:357–63 [ Google Scholar ]
- 39. World Health Organization (WHO) Preventing Disease Through Healthy Environments: Towards an Estimate of the Environmental Burden of Disease. 1106 p. Available online at: https://www.who.int/quantifying_ehimpacts/publications/preventingdisease.pdf (accessed September 22, 2019).
- 40. Stansfeld SA. Noise effects on health in the context of air pollution exposure. Int J Environ Res Public Health. (2015) 12:12735–60. 10.3390/ijerph121012735 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Ethical Unicorn. Everything You Need To Know About Aerosols & Air Pollution. (2019). Available online at: https://ethicalunicorn.com/2019/04/29/everything-you-need-to-know-about-aerosols-air-pollution/ (accessed October 4, 2019).
- 42. Colbeck I, Lazaridis M. Aerosols and environmental pollution. Sci Nat. (2009) 97:117–31. 10.1007/s00114-009-0594-x [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Incecik S, Gertler A, Kassomenos P. Aerosols and air quality. Sci Total Env. (2014) 355, 488–9. 10.1016/j.scitotenv.2014.04.012 [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. D'Amato G, Pawankar R, Vitale C, Maurizia L. Climate change and air pollution: effects on respiratory allergy. Allergy Asthma Immunol Res. (2016) 8:391–5. 10.4168/aair.2016.8.5.391 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Bezirtzoglou C, Dekas K, Charvalos E. Climate changes, environment and infection: facts, scenarios and growing awareness from the public health community within Europe. Anaerobe. (2011) 17:337–40. 10.1016/j.anaerobe.2011.05.016 [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Castelli F, Sulis G. Migration and infectious diseases. Clin Microbiol Infect. (2017) 23:283–9. 10.1016/j.cmi.2017.03.012 [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Watson JT, Gayer M, Connolly MA. Epidemics after natural disasters. Emerg Infect Dis. (2007) 13:1–5. 10.3201/eid1301.060779 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Fenn B. Malnutrition in Humanitarian Emergencies. Available online at: https://www.who.int/diseasecontrol_emergencies/publications/idhe_2009_london_malnutrition_fenn.pdf . (accessed August 15, 2017).
- 49. Lindh E, Argentini C, Remoli ME, Fortuna C, Faggioni G, Benedetti E, et al. The Italian 2017 outbreak Chikungunya virus belongs to an emerging Aedes albopictus –adapted virus cluster introduced from the Indian subcontinent. Open Forum Infect Dis. (2019) 6:ofy321. 10.1093/ofid/ofy321 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Calba C, Guerbois-Galla M, Franke F, Jeannin C, Auzet-Caillaud M, Grard G, Pigaglio L, Decoppet A, et al. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Eur Surveill. (2017) 22:17-00647. 10.2807/1560-7917.ES.2017.22.39.17-00647 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Menne B, Murray V. Floods in the WHO European Region: Health Effects and Their Prevention. Copenhagen: WHO; Weltgesundheits organisation, Regionalbüro für Europa; (2013). Available online at: http://www.euro.who.int/data/assets/pdf_file/0020/189020/e96853.pdf (accessed 15 August 2017). [ Google Scholar ]
- 52. Schneider SH. The greenhouse effect: science and policy. Science. (1989) 243:771–81. 10.1126/science.243.4892.771 [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Wilson WE, Suh HH. Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. J Air Waste Manag Assoc. (1997) 47:1238–49. 10.1080/10473289.1997.10464074 [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. US EPA (US Environmental Protection Agency) (2018). Available online at: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed September 22, 2018).
- 55. Cheung K, Daher N, Kam W, Shafer MM, Ning Z, Schauer JJ, et al. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10–2.5) in the Los Angeles area. Atmos Environ. (2011) 45:2651–62. 10.1016/j.atmosenv.2011.02.066 [ DOI ] [ Google Scholar ]
- 56. Zhang L, Yang Y, Li Y, Qian ZM, Xiao W, Wang X, et al. Short-term and long-term effects of PM2.5 on acute nasopharyngitis in 10 communities of Guangdong, China. Sci Total Env. (2019) 688:136–42. 10.1016/j.scitotenv.2019.05.470. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Kloog I, Ridgway B, Koutrakis P, Coull BA, Schwartz JD. Long- and short-term exposure to PM2.5 and mortality using novel exposure models, Epidemiology. (2013) 24:555–61. 10.1097/EDE.0b013e318294beaa [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. New Hampshire Department of Environmental Services Current and Forecasted Air Quality in New Hampshire. Environmental Fact Sheet (2019). Available online at: https://www.des.nh.gov/organization/commissioner/pip/factsheets/ard/documents/ard-16.pdf (accessed September 22, 2019).
- 59. Kappos AD, Bruckmann P, Eikmann T, Englert N, Heinrich U, Höppe P, et al. Health effects of particles in ambient air. Int J Hyg Environ Health. (2004) 207:399–407. 10.1078/1438-4639-00306 [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Boschi N. (Ed.). Defining an educational framework for indoor air sciences education. In: Education and Training in Indoor Air Sciences. Luxembourg: Springer Science & Business Media; (2012). 245 p. [ Google Scholar ]
- 61. Heal MR, Kumar P, Harrison RM. Particles, air quality, policy and health. Chem Soc Rev. (2012) 41:6606–30. 10.1039/c2cs35076a [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Bezirtzoglou E, Alexopoulos A. Ozone history and ecosystems: a goliath from impacts to advance industrial benefits and interests, to environmental and therapeutical strategies. In: Ozone Depletion, Chemistry and Impacts. (2009). p. 135–45. [ Google Scholar ]
- 63. Villányi V, Turk B, Franc B, Csintalan Z. Ozone Pollution and its Bioindication. In: Villányi V, editor. Air Pollution. London: Intech Open; (2010). 10.5772/10047 [ DOI ] [ Google Scholar ]
- 64. Massachusetts Department of Public Health Massachusetts State Health Assessment. Boston, MA (2017). Available online at: https://www.mass.gov/files/documents/2017/11/03/2017%20MA%20SHA%20final%20compressed.pdf (accessed October 30, 2017).
- 65. Lorenzini G, Saitanis C. Ozone: A Novel Plant “Pathogen.” In: Sanitá di, Toppi L, Pawlik-Skowrońska B, editors. Abiotic Stresses in Plant Springer Link (2003). p. 205–29. 10.1007/978-94-017-0255-3_8 [ DOI ] [ Google Scholar ]
- 66. Fares S, Vargas R, Detto M, Goldstein AH, Karlik J, Paoletti E, et al. Tropospheric ozone reduces carbon assimilation in trees: estimates from analysis of continuous flux measurements. Glob Change Biol. (2013) 19:2427–43. 10.1111/gcb.12222 [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Harmens H, Mills G, Hayes F, Jones L, Norris D, Fuhrer J. Air Pollution and Vegetation. ICP Vegetation Annual Report 2006/2007. (2012) [ Google Scholar ]
- 68. Emberson LD, Pleijel H, Ainsworth EA, den Berg M, Ren W, Osborne S, et al. Ozone effects on crops and consideration in crop models. Eur J Agron. (2018) 100:19–34. 10.1016/j.eja.2018.06.002 [ DOI ] [ Google Scholar ]
- 69. Alexopoulos A, Plessas S, Ceciu S, Lazar V, Mantzourani I, Voidarou C, et al. Evaluation of ozone efficacy on the reduction of microbial population of fresh cut lettuce ( Lactuca sativa ) and green bell pepper ( Capsicum annuum ). Food Control. (2013) 30:491–6. 10.1016/j.foodcont.2012.09.018 [ DOI ] [ Google Scholar ]
- 70. Alexopoulos A, Plessas S, Kourkoutas Y, Stefanis C, Vavias S, Voidarou C, et al. Experimental effect of ozone upon the microbial flora of commercially produced dairy fermented products. Int J Food Microbiol. (2017) 246:5–11. 10.1016/j.ijfoodmicro.2017.01.018 [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Maggio A, Fagnano M. Ozone damages to mediterranean crops: physiological responses. Ital J Agron. (2008) 13–20. 10.4081/ija.2008.13 [ DOI ] [ Google Scholar ]
- 72. McCarthy JT, Pelle E, Dong K, Brahmbhatt K, Yarosh D, Pernodet N. Effects of ozone in normal human epidermal keratinocytes. Exp Dermatol. (2013) 22:360–1. 10.1111/exd.12125 [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. WHO Health Risks of Ozone From Long-Range Transboundary Air Pollution. Available online at: http://www.euro.who.int/data/assets/pdf_file/0005/78647/E91843.pdf (accessed August 15, 2019).
- 74. Thiele JJ, Traber MG, Tsang K, Cross CE, Packer L. In vivo exposure to ozone depletes vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic Biol Med. (1997) 23:365–91. 10.1016/S0891-5849(96)00617-X [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, et al. Ozone dose and effect in humans and rats. A comparison using oxygen- 18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. (1994) 150:676–83. 10.1164/ajrccm.150.3.8087337 [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Lippmann M. Health effects of ozone. A critical review. JAPCA. (1989) 39:672–95. 10.1080/08940630.1989.10466554 [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, et al. Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am J Respir Crit Care Med. (2004) 170:1080–7. 10.1164/rccm.200403-333OC [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Soon W, Baliunas SL, Robinson AB, Robinson ZW. Environmental effects of increased atmospheric carbon dioxide. Climate Res. (1999) 13:149–64 10.1260/0958305991499694 [ DOI ] [ Google Scholar ]
- 79. Richmont-Bryant J, Owen RC, Graham S, Snyder M, McDow S, Oakes M, et al. Estimation of on-road NO2 concentrations, NO2/NOX ratios, and related roadway gradients from near-road monitoring data. Air Qual Atm Health. (2017) 10:611–25. 10.1007/s11869-016-0455-7 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (NO 2 ) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol. (2009) 39:743–81. 10.3109/10408440903294945 [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Chen T-M, Gokhale J, Shofer S, Kuschner WG. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci. (2007) 333:249–56. 10.1097/MAJ.0b013e31803b900f [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. US EPA. Table of Historical SO 2 NAAQS, Sulfur US EPA. Available online at: https://www3.epa.gov/ttn/naaqs/standards/so2/s_so2_history.html (accessed October 5, 2019).
- 83. WHO Regional Office of Europe (2000). Available online at: https://euro.who.int/_data/assets/pdf_file/0020/123086/AQG2ndEd_7_4Sulfuroxide.pdf
- 84. Pruss-Ustun A, Fewrell L, Landrigan PJ, Ayuso-Mateos JL. Lead exposure. Comparative Quantification of Health Risks. World Health Organization. p. 1495–1542. Available online at: https://www.who.int/publications/cra/chapters/volume2/1495-1542.pdf?ua=1
- 85. Goyer RA. Transplacental transport of lead. Environ Health Perspect. (1990) 89:101–5. 10.1289/ehp.9089101 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. National Institute of Environmental Health Sciences (NIH) Lead and Your Health. (2013). 1–4 p. Available online at: https://www.niehs.nih.gov/health/materials/lead_and_your_health_508.pdf (accessed September 17, 2019).
- 87. Farhat A, Mohammadzadeh A, Balali-Mood M, Aghajanpoor-Pasha M, Ravanshad Y. Correlation of blood lead level in mothers and exclusively breastfed infants: a study on infants aged less than six months. Asia Pac J Med Toxicol. (2013) 2:150–2. [ Google Scholar ]
- 88. Assi MA, Hezmee MNM, Haron AW, Sabri MYM, Rajion MA. The detrimental effects of lead on human and animal health. Vet World. (2016) 9:660–71. 10.14202/vetworld.2016.660-671 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet. (2016) 25:107–23. 10.1016/j.ejpe.2015.03.011 [ DOI ] [ Google Scholar ]
- 90. Kumar A, Singh BP, Punia M, Singh D, Kumar K, Jain VK. Assessment of indoor air concentrations of VOCs and their associated health risks in the library of Jawaharlal Nehru University, New Delhi. Environ Sci Pollut Res Int. (2014) 21:2240–8. 10.1007/s11356-013-2150-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Molhave L, Clausen G, Berglund B, Ceaurriz J, Kettrup A, Lindvall T, et al. Total Volatile Organic Compounds (TVOC) in Indoor Air Quality Investigations. Indoor Air. 7:225–240. 10.1111/j.1600-0668.1997.00002.x [ DOI ] [ Google Scholar ]
- 92. Gibb T. Indoor Air Quality May be Hazardous to Your Health. MSU Extension. Available online at: https://www.canr.msu.edu/news/indoor_air_quality_may_be_hazardous_to_your_health (accessed October 5, 2019).
- 93. Ebersviller S, Lichtveld K, Sexton KG, Zavala J, Lin Y-H, Jaspers I, et al. Gaseous VOCs rapidly modify particulate matter and its biological effects – Part 1: simple VOCs and model PM. Atmos Chem Phys Discuss. (2012) 12:5065–105. 10.5194/acpd-12-5065-2012 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. WHO (World Health Organization) . Dioxins and Their Effects on Human Health. Available online at: https://www.who.int/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health (accessed October 5, 2019).
- 95. EEA (European Environmental Agency) Air Quality Standards to the European Union and WHO. Available online at: https://www.eea.europa.eu/themes/data-and-maps/figures/air-quality-standards-under-the
- 96. Nakano T, Otsuki T. [Environmental air pollutants and the risk of cancer]. (Japanese). Gan To Kagaku Ryoho. (2013) 40:1441–5. [ PubMed ] [ Google Scholar ]
- 97. Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Curr Opin Pulm Med. (2016) 22:138–43. 10.1097/MCP.0000000000000248 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. (2014) 383:1581–92. 10.1016/S0140-6736(14)60617-6 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Jiang X-Q, Mei X-D, Feng D. Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis. (2016) 8:E31–40. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Bourdrel T, Bind M-A, Béjot Y, Morel O, Argacha J-F. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. (2017) 110:634–42. 10.1016/j.acvd.2017.05.003 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. (2007) 116:489–496. 10.1161/CIRCULATIONAHA.107.693622 [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens. (2011) 2011:495349. 10.4061/2011/495349 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Leary PJ, Kaufman JD, Barr RG, Bluemke DA, Curl CL, Hough CL, et al. Traffic- related air pollution and the right ventricle. the multi-ethnic study of atherosclerosis. Am J Respir Crit Care Med. (2014) 189:1093–100. 10.1164/rccm.201312-2298OC [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. (2012) 2012:782462. 10.1155/2012/782462 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Calderon-Garciduenas L, Azzarelli B, Acuna H, et al. Air pollution and brain damage. Toxicol Pathol. (2002) 30:373–89. 10.1080/01926230252929954 [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Rückerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. (2007) 115:1072–80. 10.1289/ehp.10021 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 107. Peters A, Veronesi B, Calderón-Garcidueñas L, Gehr P, Chen LC, Geiser M, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. (2006) 3:13–8. 10.1186/1743-8977-3-13 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Kelly FJ. Dietary antioxidants and environmental stress. Proc Nutr Soc. (2004) 63:579–85. 10.1079/PNS2004388 [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Bellinger DC. Very low lead exposures and children's neurodevelopment. Curr Opin Pediatr. (2008) 20:172–7. 10.1097/MOP.0b013e3282f4f97b [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Balbo P, Silvestri M, Rossi GA, Crimi E, Burastero SE. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2001) 31:625–36. 10.1046/j.1365-2222.2001.01068.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Drakaki E, Dessinioti C, Antoniou C. Air pollution and the skin. Front Environ Sci Eng China. (2014) 15:2–8. 10.3389/fenvs.2014.00011 [ DOI ] [ Google Scholar ]
- 112. Weisskopf MG, Kioumourtzoglou M-A, Roberts AL. Air pollution and autism spectrum disorders: causal or confounded? Curr Environ Health Rep. (2015) 2:430–9. 10.1007/s40572-015-0073-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 113. Mo Z, Fu Q, Lyu D, Zhang L, Qin Z, Tang Q, et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: a case-crossover study. Environ Pollut. (2019) 246:183–9. 10.1016/j.envpol.2018.11.109 [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Klopfer J. Effects of environmental air pollution on the eye. J Am Optom Assoc. (1989) 60:773–8. [ PubMed ] [ Google Scholar ]
- 115. Ashfaq A, Sharma P. Environmental effects of air pollution and application of engineered methods to combat the problem. J Indust Pollut Control. (2012) 29. [ Google Scholar ]
- 116. Madronich S, de Gruijl F. Skin cancer and UV radiation. Nature. (1993) 366:23–9. 10.1038/366023a0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Teramura A. Effects of UV-B radiation on the growth and yield of crop plants. Physiol Plant. (2006) 58:415–27. 10.1111/j.1399-3054.1983.tb04203.x [ DOI ] [ Google Scholar ]
- 118. Singh E, Tiwari S, Agrawal M. Effects of elevated ozone on photosynthesis and stomatal conductance of two soybean varieties: a case study to assess impacts of one component of predicted global climate change. Plant Biol Stuttg Ger. (2009) 11(Suppl. 1):101–8. 10.1111/j.1438-8677.2009.00263.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Manderson L. How global Warming is Adding to the Health Risks of Poor People. The Conversation. University of the Witwatersrand. Available online at: http://theconversation.com/how-global-warming-is-adding-to-the-health-risks-of-poor-people-109520 (accessed October 5, 2019).
- 120. Ministers of Energy and Environment Federal/Provincial/Territorial Ministers of Energy and Environment (Canada), editor. The Canada-Wide Acid Rain Strategy for Post-2000. Halifax: The Ministers; (1999). 11 p. [ Google Scholar ]
- 121. Zuhara S, Isaifan R. The impact of criteria air pollutants on soil and water: a review. (2018) 278–84. 10.30799/jespr.133.18040205 [ DOI ] [ Google Scholar ]
- 122. WHO . First WHO Global Conference on Air Pollution and Health. (2018). Available online at: https://www.who.int/airpollution/events/conference/en/ (accessed October 6, 2019).
- 123. What is the Kyoto Protocol? UNFCCC. Available online at: https://unfccc.int/kyoto__protocol (accessed October 6, 2019).
- 124. CopenhagenClimate Change Conference (UNFCCC). Available online at: https://unfccc.int/process-and-meetings/conferences/past-conferences/copenhagen-climate-change-conference-december-2009/copenhagen-climate-change-conference-december-2009 (accessed October 6, 2019).
- 125. Durban Climate Change Conference,. UNFCCC (2011). Available online at: https://unfccc.int/process-and-meetings/conferences/past-conferences/copenhagen-climate-change-conference-december-2009/copenhagen-climate-change-conference-december-2009 (accessed October 6, 2019).
- 126. Paris Climate Change Agreement,. (2016). Available online at: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement
- View on publisher site
- PDF (689.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Research article
- Open access
- Published: 16 November 2020
Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews
- Pawel Posadzki 1 , 2 ,
- Dawid Pieper ORCID: orcid.org/0000-0002-0715-5182 3 ,
- Ram Bajpai 4 ,
- Hubert Makaruk 5 ,
- Nadja Könsgen 3 ,
- Annika Lena Neuhaus 3 &
- Monika Semwal 6
BMC Public Health volume 20 , Article number: 1724 ( 2020 ) Cite this article
52k Accesses
205 Citations
183 Altmetric
Metrics details
Sedentary lifestyle is a major risk factor for noncommunicable diseases such as cardiovascular diseases, cancer and diabetes. It has been estimated that approximately 3.2 million deaths each year are attributable to insufficient levels of physical activity. We evaluated the available evidence from Cochrane systematic reviews (CSRs) on the effectiveness of exercise/physical activity for various health outcomes.
Overview and meta-analysis. The Cochrane Library was searched from 01.01.2000 to issue 1, 2019. No language restrictions were imposed. Only CSRs of randomised controlled trials (RCTs) were included. Both healthy individuals, those at risk of a disease, and medically compromised patients of any age and gender were eligible. We evaluated any type of exercise or physical activity interventions; against any types of controls; and measuring any type of health-related outcome measures. The AMSTAR-2 tool for assessing the methodological quality of the included studies was utilised.
Hundred and fifty CSRs met the inclusion criteria. There were 54 different conditions. Majority of CSRs were of high methodological quality. Hundred and thirty CSRs employed meta-analytic techniques and 20 did not. Limitations for studies were the most common reasons for downgrading the quality of the evidence. Based on 10 CSRs and 187 RCTs with 27,671 participants, there was a 13% reduction in mortality rates risk ratio (RR) 0.87 [95% confidence intervals (CI) 0.78 to 0.96]; I 2 = 26.6%, [prediction interval (PI) 0.70, 1.07], median effect size (MES) = 0.93 [interquartile range (IQR) 0.81, 1.00]. Data from 15 CSRs and 408 RCTs with 32,984 participants showed a small improvement in quality of life (QOL) standardised mean difference (SMD) 0.18 [95% CI 0.08, 0.28]; I 2 = 74.3%; PI -0.18, 0.53], MES = 0.20 [IQR 0.07, 0.39]. Subgroup analyses by the type of condition showed that the magnitude of effect size was the largest among patients with mental health conditions.
There is a plethora of CSRs evaluating the effectiveness of physical activity/exercise. The evidence suggests that physical activity/exercise reduces mortality rates and improves QOL with minimal or no safety concerns.
Trial registration
Registered in PROSPERO ( CRD42019120295 ) on 10th January 2019.
Peer Review reports
The World Health Organization (WHO) defines physical activity “as any bodily movement produced by skeletal muscles that requires energy expenditure” [ 1 ]. Therefore, physical activity is not only limited to sports but also includes walking, running, swimming, gymnastics, dance, ball games, and martial arts, for example. In the last years, several organizations have published or updated their guidelines on physical activity. For example, the Physical Activity Guidelines for Americans, 2nd edition, provides information and guidance on the types and amounts of physical activity that provide substantial health benefits [ 2 ]. The evidence about the health benefits of regular physical activity is well established and so are the risks of sedentary behaviour [ 2 ]. Exercise is dose dependent, meaning that people who achieve cumulative levels several times higher than the current recommended minimum level have a significant reduction in the risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events [ 3 ]. Benefits of physical activity have been reported for numerous outcomes such as mortality [ 4 , 5 ], cognitive and physical decline [ 5 , 6 , 7 ], glycaemic control [ 8 , 9 ], pain and disability [ 10 , 11 ], muscle and bone strength [ 12 ], depressive symptoms [ 13 ], and functional mobility and well-being [ 14 , 15 ]. Overall benefits of exercise apply to all bodily systems including immunological [ 16 ], musculoskeletal [ 17 ], respiratory [ 18 ], and hormonal [ 19 ]. Specifically for the cardiovascular system, exercise increases fatty acid oxidation, cardiac output, vascular smooth muscle relaxation, endothelial nitric oxide synthase expression and nitric oxide availability, improves plasma lipid profiles [ 15 ] while at the same time reducing resting heart rate and blood pressure, aortic valve calcification, and vascular resistance [ 20 ].
However, the degree of all the above-highlighted benefits vary considerably depending on individual fitness levels, types of populations, age groups and the intensity of different physical activities/exercises [ 21 ]. The majority of guidelines in different countries recommend a goal of 150 min/week of moderate-intensity aerobic physical activity (or equivalent of 75 min of vigorous-intensity) [ 22 ] with differences for cardiovascular disease [ 23 ] or obesity prevention [ 24 ] or age groups [ 25 ].
There is a plethora of systematic reviews published by the Cochrane Library critically evaluating the effectiveness of physical activity/exercise for various health outcomes. Cochrane systematic reviews (CSRs) are known to be a source of high-quality evidence. Thus, it is not only timely but relevant to evaluate the current knowledge, and determine the quality of the evidence-base, and the magnitude of the effect sizes given the negative lifestyle changes and rising physical inactivity-related burden of diseases. This overview will identify the breadth and scope to which CSRs have appraised the evidence for exercise on health outcomes; and this will help in directing future guidelines and identifying current gaps in the literature.
The objectives of this research were to a. answer the following research questions: in children, adolescents and adults (both healthy and medically compromised) what are the effects (and adverse effects) of exercise/physical activity in improving various health outcomes (e.g., pain, function, quality of life) reported in CSRs; b. estimate the magnitude of the effects by pooling the results quantitatively; c. evaluate the strength and quality of the existing evidence; and d. create recommendations for future researchers, patients, and clinicians.
Our overview was registered with PROSPERO (CRD42019120295) on 10th January 2019. The Cochrane Handbook for Systematic Reviews of interventions and Preferred Reporting Items for Overviews of Reviews were adhered to while writing and reporting this overview [ 26 , 27 ].
Search strategy and selection criteria
We followed the practical guidance for conducting overviews of reviews of health care interventions [ 28 ] and searched the Cochrane Database of Systematic Reviews (CDSR), 2019, Issue 1, on the Cochrane Library for relevant papers using the search strategy: (health) and (exercise or activity or physical). The decision to seek CSRs only was based on three main aspects. First, high quality (CSRs are considered to be the ‘gold methodological standard’) [ 29 , 30 , 31 ]. Second, data saturation (enough high-quality evidence to reach meaningful conclusions based on CSRs only). Third, including non-CSRs would have heavily increased the issue of overlapping reviews (also affecting data robustness and credibility of conclusions). One reviewer carried out the searches. The study screening and selection process were performed independently by two reviewers. We imported all identified references into reference manager software EndNote (X8). Any disagreements were resolved by discussion between the authors with third overview author acting as an arbiter, if necessary.
We included CSRs of randomised controlled trials (RCTs) involving both healthy individuals and medically compromised patients of any age and gender. Only CSRs assessing exercise or physical activity as a stand-alone intervention were included. This included interventions that could initially be taught by a professional or involve ongoing supervision (the WHO definition). Complex interventions e.g., assessing both exercise/physical activity and behavioural changes were excluded if the health effects of the interventions could not have been attributed to exercise distinctly.
Any types of controls were admissible. Reviews evaluating any type of health-related outcome measures were deemed eligible. However, we excluded protocols or/and CSRs that have been withdrawn from the Cochrane Library as well as reviews with no included studies.
Data analysis
Three authors (HM, ALN, NK) independently extracted relevant information from all the included studies using a custom-made data collection form. The methodological quality of SRs included was independently evaluated by same reviewers using the AMSTAR-2 tool [ 32 ]. Any disagreements on data extraction or CSR quality were resolved by discussion. The entire dataset was validated by three authors (PP, MS, DP) and any discrepant opinions were settled through discussions.
The results of CSRs are presented in a narrative fashion using descriptive tables. Where feasible, we presented outcome measures across CSRs. Data from the subset of homogeneous outcomes were pooled quantitatively using the approach previously described by Bellou et al. and Posadzki et al. [ 33 , 34 ]. For mortality and quality of life (QOL) outcomes, the number of participants and RCTs involved in the meta-analysis, summary effect sizes [with 95% confidence intervals (CI)] using random-effects model were calculated. For binary outcomes, we considered relative risks (RRs) as surrogate measures of the corresponding odds ratio (OR) or risk ratio/hazard ratio (HR). To stabilise the variance and normalise the distributions, we transformed RRs into their natural logarithms before pooling the data (a variation was allowed, however, it did not change interpretation of results) [ 35 ]. The standard error (SE) of the natural logarithm of RR was derived from the corresponding CIs, which was either provided in the study or calculated with standard formulas [ 36 ]. Binary outcomes reported as risk difference (RD) were also meta-analysed if two more estimates were available. For continuous outcomes, we only meta-analysed estimates that were available as standardised mean difference (SMD), and estimates reported with mean differences (MD) for QOL were presented separately in a supplementary Table 9 . To estimate the overall effect size, each study was weighted by the reciprocal of its variance. Random-effects meta-analysis, using DerSimonian and Laird method [ 37 ] was applied to individual CSR estimates to obtain a pooled summary estimate for RR or SMD. The 95% prediction interval (PI) was also calculated (where ≥3 studies were available), which further accounts for between-study heterogeneity and estimates the uncertainty around the effect that would be anticipated in a new study evaluating that same association. I -squared statistic was used to measure between study heterogeneity; and its various thresholds (small, substantial and considerable) were interpreted considering the size and direction of effects and the p -value from Cochran’s Q test ( p < 0.1 considered as significance) [ 38 ]. Wherever possible, we calculated the median effect size (with interquartile range [IQR]) of each CSR to interpret the direction and magnitude of the effect size. Sub-group analyses are planned for type and intensity of the intervention; age group; gender; type and/or severity of the condition, risk of bias in RCTs, and the overall quality of the evidence (Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria). To assess overlap we calculated the corrected covered area (CCA) [ 39 ]. All statistical analyses were conducted on Stata statistical software version 15.2 (StataCorp LLC, College Station, Texas, USA).
The searches generated 280 potentially relevant CRSs. After removing of duplicates and screening, a total of 150 CSRs met our eligibility criteria [ 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 ] (Fig. 1 ). Reviews were published between September 2002 and December 2018. A total of 130 CSRs employed meta-analytic techniques and 20 did not. The total number of RCTs in the CSRs amounted to 2888; with 485,110 participants (mean = 3234, SD = 13,272). The age ranged from 3 to 87 and gender distribution was inestimable. The main characteristics of included reviews are summarised in supplementary Table 1 . Supplementary Table 2 summarises the effects of physical activity/exercise on health outcomes. Conclusions from CSRs are listed in supplementary Table 3 . Adverse effects are listed in supplementary Table 4 . Supplementary Table 5 presents summary of withdrawals/non-adherence. The methodological quality of CSRs is presented in supplementary Table 6 . Supplementary Table 7 summarises studies assessed at low risk of bias (by the authors of CSRs). GRADE-ings of the review’s main comparison are listed in supplementary Table 8 .
Study selection process
There were 54 separate populations/conditions, considerable range of interventions and comparators, co-interventions, and outcome measures. For detailed description of interventions, please refer to the supplementary tables . Most commonly measured outcomes were - function 112 (75%), QOL 83 (55%), AEs 70 (47%), pain 41 (27%), mortality 28 (19%), strength 30 (20%), costs 47 (31%), disability 14 (9%), and mental health in 35 (23%) CSRs.
There was a 13% reduction in mortality rates risk ratio (RR) 0.87 [95% CI 0.78 to 0.96]; I 2 = 26.6%, [PI 0.70, 1.07], median effect size (MES) = 0.93 [interquartile range (IQR) 0.81, 1.00]; 10 CSRs, 187 RCTs, 27,671 participants) following exercise when compared with various controls (Table 1 ). This reduction was smaller in ‘other groups’ of patients when compared to cardiovascular diseases (CVD) patients - RR 0.97 [95% CI 0.65, 1.45] versus 0.85 [0.76, 0.96] respectively. The effects of exercise were not intensity or frequency dependent. Sessions more than 3 times per week exerted a smaller reduction in mortality as compared with sessions of less than 3 times per week RR 0.87 [95% CI 0.78, 0.98] versus 0.63 [0.39, 1.00]. Subgroup analyses by risk of bias (ROB) in RCTs showed that RCTs at low ROB exerted smaller reductions in mortality when compared to RCTs at an unclear or high ROB, RR 0.90 [95% CI 0.78, 1.02] versus 0.72 [0.42, 1.22] versus 0.86 [0.69, 1.06] respectively. CSRs with moderate quality of evidence (GRADE), showed slightly smaller reductions in mortality when compared with CSRs that relied on very low to low quality evidence RR 0.88 [95% CI 0.79, 0.98] versus 0.70 [0.47, 1.04].
Exercise also showed an improvement in QOL, standardised mean difference (SMD) 0.18 [95% CI 0.08, 0.28]; I 2 = 74.3%; PI -0.18, 0.53], MES = 0.20 [IQR 0.07, 0.39]; 15 CSRs, 408 RCTs, 32,984 participants) when compared with various controls (Table 2 ). These improvements were greater observed for health related QOL when compared to overall QOL SMD 0.30 [95% CI 0.21, 0.39] vs 0.06 [− 0.08, 0.20] respectively. Again, the effects of exercise were duration and frequency dependent. For instance, sessions of more than 90 mins exerted a greater improvement in QOL as compared with sessions up to 90 min SMD 0.24 [95% CI 0.11, 0.37] versus 0.22 [− 0.30, 0.74]. Subgroup analyses by the type of condition showed that the magnitude of effect was the largest among patients with mental health conditions, followed by CVD and cancer. Physical activity exerted negative effects on QOL in patients with respiratory conditions (2 CSRs, 20 RCTs with 601 patients; SMD -0.97 [95% CI -1.43, 0.57]; I 2 = 87.8%; MES = -0.46 [IQR-0.97, 0.05]). Subgroup analyses by risk of bias (ROB) in RCTs showed that RCTs at low or unclear ROB exerted greater improvements in QOL when compared to RCTs at a high ROB SMD 0.21 [95% CI 0.10, 0.31] versus 0.17 [0.03, 0.31]. Analogically, CSRs with moderate to high quality of evidence showed slightly greater improvements in QOL when compared with CSRs that relied on very low to low quality evidence SMD 0.19 [95% CI 0.05, 0.33] versus 0.15 [− 0.02, 0.32]. Please also see supplementary Table 9 more studies reporting QOL outcomes as mean difference (not quantitatively synthesised herein).
Adverse events (AEs) were reported in 100 (66.6%) CSRs; and not reported in 50 (33.3%). The number of AEs ranged from 0 to 84 in the CSRs. The number was inestimable in 83 (55.3%) CSRs. Ten (6.6%) reported no occurrence of AEs. Mild AEs were reported in 28 (18.6%) CSRs, moderate in 9 (6%) and serious/severe in 20 (13.3%). There were 10 deaths and in majority of instances, the causality was not attributed to exercise. For this outcome, we were unable to pool the data as effect sizes were too heterogeneous (Table 3 ).
In 38 CSRs, the total number of trials reporting withdrawals/non-adherence was inestimable. There were different ways of reporting it such as adherence or attrition (high in 23.3% of CSRs) as well as various effect estimates including %, range, total numbers, MD, RD, RR, OR, mean and SD. The overall pooled estimates are reported in Table 3 .
Of all 16 domains of the AMSTAR-2 tool, 1876 (78.1%) scored ‘yes’, 76 (3.1%) ‘partial yes’; 375 (15.6%) ‘no’, and ‘not applicable’ in 25 (1%) CSRs. Ninety-six CSRs (64%) were scored as ‘no’ on reporting sources of funding for the studies followed by 88 (58.6%) failing to explain the selection of study designs for inclusion. One CSR (0.6%) each were judged as ‘no’ for reporting any potential sources of conflict of interest, including any funding for conducting the review as well for performing study selection in duplicate.
In 102 (68%) CSRs, there was predominantly a high risk of bias in RCTs. In 9 (6%) studies, this was reported as a range, e.g., low or unclear or low to high. Two CSRs used different terminology i.e., moderate methodological quality; and the risk of bias was inestimable in one CSR. Sixteen (10.6%) CSRs did not identify any studies (RCTs) at low risk of random sequence generation, 28 (18.6%) allocation concealment, 28 (18.6%) performance bias, 84 (54%) detection bias, 35 (23.3%) attrition bias, 18 (12%) reporting bias, and 29 (19.3%) other bias.
In 114 (76%) CSRs, limitation of studies was the main reason for downgrading the quality of the evidence followed by imprecision in 98 (65.3%) and inconsistency in 68 (45.3%). Publication bias was the least frequent reason for downgrading in 26 (17.3%) CSRs. Ninety-one (60.7%) CSRs reached equivocal conclusions, 49 (32.7%) reviews reached positive conclusions and 10 (6.7%) reached negative conclusions (as judged by the authors of CSRs).
In this systematic review of CSRs, we found a large body of evidence on the beneficial effects of physical activity/exercise on health outcomes in a wide range of heterogeneous populations. Our data shows a 13% reduction in mortality rates among 27,671 participants, and a small improvement in QOL and health-related QOL following various modes of physical activity/exercises. This means that both healthy individuals and medically compromised patients can significantly improve function, physical and mental health; or reduce pain and disability by exercising more [ 190 ]. In line with previous findings [ 191 , 192 , 193 , 194 ], where a dose-specific reduction in mortality has been found, our data shows a greater reduction in mortality in studies with longer follow-up (> 12 months) as compared to those with shorter follow-up (< 12 months). Interestingly, we found a consistent pattern in the findings, the higher the quality of evidence and the lower the risk of bias in primary studies, the smaller reductions in mortality. This pattern is observational in nature and cannot be over-generalised; however this might mean less certainty in the estimates measured. Furthermore, we found that the magnitude of the effect size was the largest among patients with mental health conditions. A possible mechanism of action may involve elevated levels of brain-derived neurotrophic factor or beta-endorphins [ 195 ].
We found the issue of poor reporting or underreporting of adherence/withdrawals in over a quarter of CSRs (25.3%). This is crucial both for improving the accuracy of the estimates at the RCT level as well as maintaining high levels of physical activity and associated health benefits at the population level.
Even the most promising interventions are not entirely risk-free; and some minor AEs such as post-exercise pain and soreness or discomfort related to physical activity/exercise have been reported. These were typically transient; resolved within a few days; and comparable between exercise and various control groups. However worryingly, the issue of poor reporting or underreporting of AEs has been observed in one third of the CSRs. Transparent reporting of AEs is crucial for identifying patients at risk and mitigating any potential negative or unintended consequences of the interventions.
High risk of bias of the RCTs evaluated was evident in more than two thirds of the CSRs. For example, more than half of reviews identified high risk of detection bias as a major source of bias suggesting that lack of blinding is still an issue in trials of behavioural interventions. Other shortcomings included insufficiently described randomisation and allocation concealment methods and often poor outcome reporting. This highlights the methodological challenges in RCTs of exercise and the need to counterbalance those with the underlying aim of strengthening internal and external validity of these trials.
Overall, high risk of bias in the primary trials was the main reason for downgrading the quality of the evidence using the GRADE criteria. Imprecision was frequently an issue, meaning the effective sample size was often small; studies were underpowered to detect the between-group differences. Pooling too heterogeneous results often resulted in inconsistent findings and inability to draw any meaningful conclusions. Indirectness and publication bias were lesser common reasons for downgrading. However, with regards to the latter, the generally accepted minimum number of 10 studies needed for quantitatively estimate the funnel plot asymmetry was not present in 69 (46%) CSRs.
Strengths of this research are the inclusion of large number of ‘gold standard’ systematic reviews, robust screening, data extractions and critical methodological appraisal. Nevertheless, some weaknesses need to be highlighted when interpreting findings of this overview. For instance, some of these CSRs analysed the same primary studies (RCTs) but, arrived at slightly different conclusions. Using, the Pieper et al. [ 39 ] formula, the amount of overlap ranged from 0.01% for AEs to 0.2% for adherence, which indicates slight overlap. All CSRs are vulnerable to publication bias [ 196 ] - hence the conclusions generated by them may be false-positive. Also, exercise was sometimes part of a complex intervention; and the effects of physical activity could not be distinguished from co-interventions. Often there were confounding effects of diet, educational, behavioural or lifestyle interventions; selection, and measurement bias were inevitably inherited in this overview too. Also, including CSRs only might lead to selection bias; and excluding reviews published before 2000 might limit the overall completeness and applicability of the evidence. A future update should consider these limitations, and in particular also including non-CSRs.

Conclusions
Trialists must improve the quality of primary studies. At the same time, strict compliance with the reporting standards should be enforced. Authors of CSRs should better explain eligibility criteria and report sources of funding for the primary studies. There are still insufficient physical activity trends worldwide amongst all age groups; and scalable interventions aimed at increasing physical activity levels should be prioritized [ 197 ]. Hence, policymakers and practitioners need to design and implement comprehensive and coordinated strategies aimed at targeting physical activity programs/interventions, health promotion and disease prevention campaigns at local, regional, national, and international levels [ 198 ].
Availability of data and materials
Data sharing is not applicable to this article as no raw data were analysed during the current study. All information in this article is based on published systematic reviews.
Abbreviations
Adverse events
Cardiovascular diseases
Cochrane Database of Systematic Reviews
Cochrane systematic reviews
Confidence interval
Grading of Recommendations Assessment, Development and Evaluation
Hazard ratio
Interquartile range
Mean difference
Prediction interval
Quality of life
Randomised controlled trials
Relative risk
Risk difference
Risk of bias
Standard error
Standardised mean difference
World Health Organization
https://www.who.int/dietphysicalactivity/pa/en/ . (Accessed 8 June 2020).
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for AmericansPhysical activity guidelines for AmericansPhysical activity guidelines for Americans. Jama. 2018;320(19):2020–8.
PubMed Google Scholar
Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the global burden of disease study 2013. BMJ. 2016;354:i3857.
PubMed PubMed Central Google Scholar
Abell B, Glasziou P, Hoffmann T. The contribution of individual exercise training components to clinical outcomes in randomised controlled trials of cardiac rehabilitation: a systematic review and meta-regression. Sports Med Open. 2017;3(1):19.
Anderson D, Seib C, Rasmussen L. Can physical activity prevent physical and cognitive decline in postmenopausal women? A systematic review of the literature. Maturitas. 2014;79(1):14–33.
Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: a systematic review. Physiother Can. 2010;62(1):25–34.
Barlow PA, Otahal P, Schultz MG, Shing CM, Sharman JE. Low exercise blood pressure and risk of cardiovascular events and all-cause mortality: systematic review and meta-analysis. Atherosclerosis. 2014;237(1):13–22.
CAS PubMed Google Scholar
Aljawarneh YM, Wardell DW, Wood GL, Rozmus CL. A systematic review of physical activity and exercise on physiological and biochemical outcomes in children and adolescents with type 1 diabetes. J Nurs Scholarsh. 2019.
Chastin SFM, De Craemer M, De Cocker K, Powell L, Van Cauwenberg J, Dall P, Hamer M, Stamatakis E. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53(6):370–6.
Abdulla SY, Southerst D, Cote P, Shearer HM, Sutton D, Randhawa K, Varatharajan S, Wong JJ, Yu H, Marchand AA, et al. Is exercise effective for the management of subacromial impingement syndrome and other soft tissue injuries of the shoulder? A systematic review by the Ontario protocol for traffic injury management (OPTIMa) collaboration. Man Ther. 2015;20(5):646–56.
Alanazi MH, Parent EC, Dennett E. Effect of stabilization exercise on back pain, disability and quality of life in adults with scoliosis: a systematic review. Eur J Phys Rehabil Med. 2018;54(5):647–53.
Adsett JA, Mudge AM, Morris N, Kuys S, Paratz JD. Aquatic exercise training and stable heart failure: a systematic review and meta-analysis. Int J Cardiol. 2015;186:22–8.
Adamson BC, Ensari I, Motl RW. Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(7):1329–38.
Abdin S, Welch RK, Byron-Daniel J, Meyrick J. The effectiveness of physical activity interventions in improving well-being across office-based workplace settings: a systematic review. Public Health. 2018;160:70–6.
Albalawi H, Coulter E, Ghouri N, Paul L. The effectiveness of structured exercise in the south Asian population with type 2 diabetes: a systematic review. Phys Sportsmed. 2017;45(4):408–17.
Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J, Bragazzi N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:2187.
Hagen KB, Dagfinrud H, Moe RH, Østerås N, Kjeken I, Grotle M, Smedslund G. Exercise therapy for bone and muscle health: an overview of systematic reviews. BMC Med. 2012;10(1):167.
Burton DA, Stokes K, Hall GM. Physiological effects of exercise. Contin Educ Anaesth Crit Care Pain. 2004;4(6):185–8.
Google Scholar
Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–61.
Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 2018;5:135.
CAS PubMed PubMed Central Google Scholar
Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167(1):1–12.
Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–56.
Excellence NIfHaC: Cardiovascular disease prevention public health guideline [PH25] Published date: June 2010. Available at: https://www.nice.org.uk/guidance/ph25/resources/cardiovascular-disease-prevention-pdf-1996238687173 .
Excellence NIfHaC: Obesity prevention clinical guideline [CG43] published date: December 2006 Last updated: March 2015. Available at: https://www.nice.org.uk/guidance/cg43/resources/obesity-prevention-pdf-975445344709 .
Excellence NIfHaC: Physical activity for children and young people public health guideline [PH17] Published date: January 2009. Available at: https://www.nice.org.uk/guidance/ph17/resources/physical-activity-for-children-and-young-people-pdf-1996181580229 .
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 6 [updated September 2018] edition. Available from www.cochrane-handbook.org : The Cochrane Collaboration, 2011. 2011.
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich A-B. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24.
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18.
Handoll H, Madhok R. Quality of Cochrane reviews. Another study found that most Cochrane reviews are of a good standard. BMJ. 2002;324(7336):545.
Petticrew M, Wilson P, Wright K, Song F. Quality of Cochrane reviews. Quality of Cochrane reviews is better than that of non-Cochrane reviews. BMJ. 2002;324(7336):545.
Shea B, Moher D, Graham I, Pham B, Tugwell P. A comparison of the quality of Cochrane reviews and systematic reviews published in paper-based journals. Eval Health Prof. 2002;25(1):116–29.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Posadzki PP, Bajpai R, Kyaw BM, Roberts NJ, Brzezinski A, Christopoulos GI, Divakar U, Bajpai S, Soljak M, Dunleavy G, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med. 2018;16(1):18.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9.
Walter SD, Cook RJ. A comparison of several point estimators of the odds ratio in a single 2 x 2 contingency table. Biometrics. 1991;47(3):795–811.
Khan H, Sempos CT. Statistical methods in epidemiology. New York: Oxford University Press; 1989.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
CAS Google Scholar
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019): Cochrane; 2019. Available from www.training.cochrane.org/handbook .
Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75.
Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev. 2012;7.
Al-Khudairy L, Loveman E, Colquitt JL, Mead E, Johnson RE, Fraser H, Olajide J, Murphy M, Velho RM, O'Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst Rev. 2017;6.
Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2013;7.
Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4.
Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;1.
Andriolo RB, El Dib RP, Ramos L, Atallah Á, da Silva EMK. Aerobic exercise training programmes for improving physical and psychosocial health in adults with Down syndrome. Cochrane Database Syst Rev. 2010;5.
Araujo DN, Ribeiro CTD, Maciel ACC, Bruno SS, Fregonezi GAF, Dias FAL. Physical exercise for the treatment of non-ulcerated chronic venous insufficiency. Cochrane Database Syst Rev. 2016;12.
Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005;1.
Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold-Samsøe B, Dagfinrud H, Lund H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3.
Beggs S, Foong YC, Le HCT, Noor D, Wood-Baker R, Walters JAE. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev. 2013;4.
Bergenthal N, Will A, Streckmann F, Wolkewitz KD, Monsef I, Engert A, Elter T, Skoetz N. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev. 2014;11.
Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Góes SM, Boden C, Foulds HJA. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;6.
Bidonde J, Busch AJ, van der Spuy I, Tupper S, Kim SY, Boden C. Whole body vibration exercise training for fibromyalgia. Cochrane Database Syst Rev. 2017;9.
Bidonde J, Busch AJ, Webber SC, Schachter CL, Danyliw A, Overend TJ, Richards RS, Rader T. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;10.
Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJL. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3.
Bradt J, Shim M, Goodill SW. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev. 2015;1.
Broderick J, Crumlish N, Waugh A, Vancampfort D. Yoga versus non-standard care for schizophrenia. Cochrane Database Syst Rev. 2017;9.
Broderick J, Knowles A, Chadwick J, Vancampfort D. Yoga versus standard care for schizophrenia. Cochrane Database Syst Rev. 2015;10.
Broderick J, Vancampfort D. Yoga as part of a package of care versus standard care for schizophrenia. Cochrane Database Syst Rev. 2017;9.
Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev. 2017;6.
Busch AJ, Barber KA, Overend TJ, Peloso PMJ, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;4.
Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, Danyliw A, Sawant A, Dal Bello-Haas V, Rader T, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;12.
Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9.
Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013;9.
Carvalho APV, Vital FMR, Soares BGO. Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database Syst Rev. 2012;4.
Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6.
Cavalheri V, Tahirah F, Nonoyama ML, Jenkins S, Hill K. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev. 2013;7.
Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database Syst Rev. 2006;3.
Choi BKL, Verbeek JH, Tam WWS, Jiang JY. Exercises for prevention of recurrences of low-back pain. Cochrane Database Syst Rev. 2010;1.
Colquitt JL, Loveman E, O'Malley C, Azevedo LB, Mead E, Al-Khudairy L, Ells LJ, Metzendorf MI, Rees K. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database Syst Rev. 2016;3.
Connolly B, Salisbury L, O'Neill B, Geneen LJ, Douiri A, Grocott MPW, Hart N, Walsh TS, Blackwood B. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev. 2015;6.
Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE. Exercise for depression. Cochrane Database Syst Rev. 2013;9.
Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev. 2015;10.
Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1.
Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11.
Dal Bello-Haas V, Florence JM. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev. 2013;5.
Dale MT, McKeough ZJ, Troosters T, Bye P, Alison JA. Exercise training to improve exercise capacity and quality of life in people with non-malignant dust-related respiratory diseases. Cochrane Database Syst Rev. 2015;11.
Daley A, Stokes-Lampard H, Thomas A, MacArthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;11.
de Morton N, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;1.
Dobbins M, Husson H, DeCorby K, LaRocca RL. School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18. Cochrane Database Syst Rev. 2013;2.
Doiron KA, Hoffmann TC, Beller EM. Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst Rev. 2018;3.
Ekeland E, Heian F, Hagen KB, Abbott JM, Nordheim L. Exercise to improve self-esteem in children and young people. Cochrane Database Syst Rev. 2004;1.
Elbers RG, Verhoef J, van Wegen EEH, Berendse HW, Kwakkel G. Interventions for fatigue in Parkinson's disease. Cochrane Database Syst Rev. 2015;10.
Felbel S, Meerpohl JJ, Monsef I, Engert A, Skoetz N. Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database Syst Rev. 2014;6.
Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4.
Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1.
Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;4.
Freitas DA, Holloway EA, Bruno SS, Chaves GSS, Fregonezi GAF, Mendonça K. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2013;10.
Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9.
Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev. 2013;1.
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9.
Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Cochrane Database Syst Rev. 2010;5.
Grande AJ, Keogh J, Hoffmann TC, Beller EM, Del Mar CB. Exercise versus no exercise for the occurrence, severity and duration of acute respiratory infections. Cochrane Database Syst Rev. 2015;6.
Grande AJ, Reid H, Thomas EE, Nunan D, Foster C. Exercise prior to influenza vaccination for limiting influenza incidence and its related complications in adults. Cochrane Database Syst Rev. 2016;8.
Grande AJ, Silva V, Andriolo BNG, Riera R, Parra SA, Peccin MS. Water-based exercise for adults with asthma. Cochrane Database Syst Rev. 2014;7.
Gross A, Kay TM, Paquin JP, Blanchette S, Lalonde P, Christie T, Dupont G, Graham N, Burnie SJ, Gelley G, et al. Exercises for mechanical neck disorders. Cochrane Database Syst Rev. 2015;1.
Hageman D, Fokkenrood HJP, Gommans LNM, van den Houten MML, Teijink JAW. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4.
Han A, Judd M, Welch V, Wu T, Tugwell P, Wells GA. Tai chi for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2004;3.
Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;7.
Hartley L, Dyakova M, Holmes J, Clarke A, Lee MS, Ernst E, Rees K. Yoga for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;5.
Hartley L, Flowers N, Lee MS, Ernst E, Rees K. Tai chi for primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;4.
Hartley L, Lee MS, Kwong JSW, Flowers N, Todkill D, Ernst E, Rees K. Qigong for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;6.
Hassett L, Moseley AM, Harmer AR. Fitness training for cardiorespiratory conditioning after traumatic brain injury. Cochrane Database Syst Rev. 2017;12.
Hayden J, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;3.
Hay-Smith EJC, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2011;12.
Heine M, van de Port I, Rietberg MB, van Wegen EEH, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2015;9.
Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;10.
Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué i Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12.
Herbert RD, de Noronha M, Kamper SJ. Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Syst Rev. 2011;7.
Heymans MW, van Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for non-specific low-back pain. Cochrane Database Syst Rev. 2004;4.
Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;10.
Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;11.
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7.
Hurkmans E, van der Giesen FJ, Vliet Vlieland TPM, Schoones J, Van den Ende E. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev. 2009;4.
Hurley M, Dickson K, Hallett R, Grant R, Hauari H, Walsh N, Stansfield C, Oliver S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;4.
Jones M, Harvey A, Marston L, O'Connell NE. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013;5.
Katsura M, Kuriyama A, Takeshima T, Fukuhara S, Furukawa TA. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev. 2015;10.
Kendrick D, Kumar A, Carpenter H, Zijlstra GAR, Skelton DA, Cook JR, Stevens Z, Belcher CM, Haworth D, Gawler SJ, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev. 2014;11.
Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;3.
Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1.
Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12.
Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2017;4.
Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3.
Lauret GJ, Fakhry F, Fokkenrood HJP, Hunink MGM, Teijink JAW, Spronk S. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev. 2014;7.
Lawrence M, Celestino Junior FT, Matozinho HHS, Govan L, Booth J, Beecher J. Yoga for stroke rehabilitation. Cochrane Database Syst Rev. 2017;12.
Lin CWC, Donkers NAJ, Refshauge KM, Beckenkamp PR, Khera K, Moseley AM. Rehabilitation for ankle fractures in adults. Cochrane Database Syst Rev. 2012;11.
Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3.
Long L, Anderson L, Dewhirst AM, He J, Bridges C, Gandhi M, Taylor RS. Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev. 2018;2.
Loughney LA, West MA, Kemp GJ, Grocott MPW, Jack S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst Rev. 2018;12.
Macedo LG, Saragiotto BT, Yamato TP, Costa LOP, Menezes Costa LC, Ostelo R, Maher CG. Motor control exercise for acute non-specific low back pain. Cochrane Database Syst Rev. 2016;2.
Macêdo TMF, Freitas DA, Chaves GSS, Holloway EA, Mendonça K. Breathing exercises for children with asthma. Cochrane Database Syst Rev. 2016;4.
Martin A, Booth JN, Laird Y, Sproule J, Reilly JJ, Saunders DH. Physical activity, diet and other behavioural interventions for improving cognition and school achievement in children and adolescents with obesity or overweight. Cochrane Database Syst Rev. 2018;3.
McKeough ZJ, Velloso M, Lima VP, Alison JA. Upper limb exercise training for COPD. Cochrane Database Syst Rev. 2016;11.
McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Water-based exercise training for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;12.
McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, Mackey J, Courneya K. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;6.
Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, Olajide J, Mainardi GM, Corpeleijn E, O'Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6.
Meekums B, Karkou V, Nelson EA. Dance movement therapy for depression. Cochrane Database Syst Rev. 2015;2.
Meher S, Duley L. Exercise or other physical activity for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2006;2.
Mehrholz J, Kugler J, Pohl M. Water-based exercises for improving activities of daily living after stroke. Cochrane Database Syst Rev. 2011;1.
Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2012;11.
Mehrholz J, Thomas S, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2017;8.
Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8.
Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8.
Montgomery P, Dennis JA. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002;4.
Morris NR, Kermeen FD, Holland AE. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst Rev. 2017;1.
Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;6.
Ngai SPC, Jones AYM, Tam WWS. Tai chi for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2016;6.
Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2012;7.
O'Brien K, Nixon S, Glazier R, Tynan AM. Progressive resistive exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2004;4.
O'Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010;8.
Østerås N, Kjeken I, Smedslund G, Moe RH, Slatkowsky-Christensen B, Uhlig T, Hagen KB. Exercise for hand osteoarthritis. Cochrane Database Syst Rev. 2017;1.
Page MJ, Green S, Kramer S, Johnston RV, McBain B, Chau M, Buchbinder R. Manual therapy and exercise for adhesive capsulitis (frozen shoulder). Cochrane Database Syst Rev. 2014;8.
Page MJ, Green S, McBain B, Surace SJ, Deitch J, Lyttle N, Mrocki MA, Buchbinder R. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;6.
Page MJ, O'Connor D, Pitt V, Massy-Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;6.
Panebianco M, Sridharan K, Ramaratnam S. Yoga for epilepsy. Cochrane Database Syst Rev. 2017;10.
Perry A, Lee SH, Cotton S, Kennedy C. Therapeutic exercises for affecting post-treatment swallowing in people treated for advanced-stage head and neck cancers. Cochrane Database Syst Rev. 2016;8.
Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database Syst Rev. 2017;11.
Regnaux JP, Lefevre-Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, Ravaud P. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev. 2015;10.
Ren J, Xia J. Dance therapy for schizophrenia. Cochrane Database Syst Rev. 2013;10.
Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005;1.
Risom SS, Zwisler AD, Johansen PP, Sibilitz KL, Lindschou J, Gluud C, Taylor RS, Svendsen JH, Berg SK. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst Rev. 2017;2.
Romano M, Minozzi S, Bettany-Saltikov J, Zaina F, Chockalingam N, Kotwicki T, Maier-Hennes A, Negrini S. Exercises for adolescent idiopathic scoliosis. Cochrane Database Syst Rev. 2012;8.
Ryan JM, Cassidy EE, Noorduyn SG, O'Connell NE. Exercise interventions for cerebral palsy. Cochrane Database Syst Rev. 2017;6.
Saragiotto BT, Maher CG, Yamato TP, Costa LOP, Menezes Costa LC, Ostelo R, Macedo LG. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016;1.
Saunders DH, Sanderson M, Hayes S, Kilrane M, Greig CA, Brazzelli M, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2016;3.
Schulzke SM, Kaempfen S, Trachsel D, Patole SK. Physical activity programs for promoting bone mineralization and growth in preterm infants. Cochrane Database Syst Rev. 2014;4.
Seron P, Lanas F, Pardo Hernandez H, Bonfill Cosp X. Exercise for people with high cardiovascular risk. Cochrane Database Syst Rev. 2014;8.
Shaw KA, Gennat HC, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4.
Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;11.
Sibilitz KL, Berg SK, Tang LH, Risom SS, Gluud C, Lindschou J, Kober L, Hassager C, Taylor RS, Zwisler AD. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev. 2016;3.
Silva IS, Fregonezi GAF, Dias FAL, Ribeiro CTD, Guerra RO, Ferreira GMH. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. 2013;9.
States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev. 2009;3.
Strike K, Mulder K, Michael R. Exercise for haemophilia. Cochrane Database Syst Rev. 2016;12.
Takken T, Van Brussel M, Engelbert RH, van der Net JJ, Kuis W, Helders P. Exercise therapy in juvenile idiopathic arthritis. Cochrane Database Syst Rev. 2008;2.
Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal H, Lough F, Rees K, Singh SJ, Mordi IR. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;4.
Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3.
Ussher MH, Taylor AH, Faulkner GEJ. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2014;8.
Valentín-Gudiol M, Mattern-Baxter K, Girabent-Farrés M, Bagur-Calafat C, Hadders-Algra M, Angulo-Barroso RM. Treadmill interventions in children under six years of age at risk of neuromotor delay. Cochrane Database Syst Rev. 2017;7.
van der Heijden RA, Lankhorst NE, van Linschoten R, Bierma-Zeinstra SMA, van Middelkoop M. Exercise for treating patellofemoral pain syndrome. Cochrane Database Syst Rev. 2015;1.
Vloothuis JDM, Mulder M, Veerbeek JM, Konijnenbelt M, Visser-Meily JMA, Ket JCF, Kwakkel G, van Wegen EEH. Caregiver-mediated exercises for improving outcomes after stroke. Cochrane Database Syst Rev. 2016;12.
Voet NBM, van der Kooi EL. Riphagen, II, Lindeman E, van Engelen BGM, Geurts ACH: strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2013;7.
White CM, Pritchard J, Turner-Stokes L. Exercise for people with peripheral neuropathy. Cochrane Database Syst Rev. 2004;4.
Wieland LS, Skoetz N, Pilkington K, Vempati R, D'Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;1.
Williams AD, Bird ML, Hardcastle SGK, Kirschbaum M, Ogden KJ, Walters JAE. Exercise for reducing falls in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;10.
Williams MA, Srikesavan C, Heine PJ, Bruce J, Brosseau L, Hoxey-Thomas N, Lamb SE. Exercise for rheumatoid arthritis of the hand. Cochrane Database Syst Rev. 2018;7.
Yamamoto S, Hotta K, Ota E, Matsunaga A, Mori R. Exercise-based cardiac rehabilitation for people with implantable ventricular assist devices. Cochrane Database Syst Rev. 2018;9.
Yamato TP, Maher CG, Saragiotto BT, Hancock MJ, Ostelo R, Cabral CMN, Menezes Costa LC, Costa LOP. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7.
Yang ZY, Zhong HB, Mao C, Yuan JQ, Huang YF, Wu XY, Gao YM, Tang JL. Yoga for asthma. Cochrane Database Syst Rev. 2016;4.
Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015;4.
Zainuldin R, Mackey MG, Alison JA. Optimal intensity and type of leg exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;11.
Mok A, Khaw K-T, Luben R, Wareham N, Brage S. Physical activity trajectories and mortality: population based cohort study. Bmj. 2019;365:l2323.
Ekelund U, Brown WJ, Steene-Johannessen J, Fagerland MW, Owen N, Powell KE, Bauman AE, Lee IM. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br J Sports Med. 2019;53(14):886–94. https://doi.org/10.1136/bjsports-2017-098963 . Epub 2018 Jul 10.
Article PubMed Google Scholar
Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–10.
Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, et al. The effect of physical activity on mortality and cardiovascular disease in 130000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–54.
Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–95.
Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–51.
Horton R. Offline: the gravy train of systematic reviews. Lancet. 2019;394(10211):1790.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6(10):e1077–86.
Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72(14):1622–39.
Download references
Acknowledgements
Not applicable.
There was no funding source for this study. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Kleijnen Systematic Reviews Ltd., York, UK
Pawel Posadzki
Nanyang Technological University, Singapore, Singapore
Institute for Research in Operative Medicine, Witten/Herdecke University, Witten, Germany
Dawid Pieper, Nadja Könsgen & Annika Lena Neuhaus
School of Medicine, Keele University, Staffordshire, UK
Jozef Pilsudski University of Physical Education in Warsaw, Faculty Physical Education and Health, Biala Podlaska, Poland
Hubert Makaruk
Health Outcomes Division, University of Texas at Austin College of Pharmacy, Austin, USA
Monika Semwal
You can also search for this author in PubMed Google Scholar
Contributions
PP wrote the protocol, ran the searches, validated, analysed and synthesised data, wrote and revised the drafts. HM, NK and ALN screened and extracted data. MS and DP validated and analysed the data. RB ran statistical analyses. All authors contributed to writing and reviewing the manuscript. PP is the guarantor. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Dawid Pieper .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Supplementary Table 1. Main characteristics of included Cochrane systematic reviews evaluating the effects of physical activity/exercise on health outcomes ( n = 150). Supplementary Table 2. Additional information from Cochrane systematic reviews of the effects of physical activity/exercise on health outcomes ( n = 150). Supplementary Table 3. Conclusions from Cochrane systematic reviews “quote”. Supplementary Table 4 . AEs reported in Cochrane systematic reviews. Supplementary Table 5. Summary of withdrawals/non-adherence. Supplementary Table 6. Methodological quality assessment of the included Cochrane reviews with AMSTAR-2. Supplementary Table 7. Number of studies assessed as low risk of bias per domain. Supplementary Table 8. GRADE for the review’s main comparison. Supplementary Table 9. Studies reporting quality of life outcomes as mean difference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Posadzki, P., Pieper, D., Bajpai, R. et al. Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health 20 , 1724 (2020). https://doi.org/10.1186/s12889-020-09855-3
Download citation
Received : 01 April 2020
Accepted : 08 November 2020
Published : 16 November 2020
DOI : https://doi.org/10.1186/s12889-020-09855-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
Published research on the human health implications of climate change between 2012 and 2021: cross sectional study
Author affiliations
Victoria L Bartlett 1
Harry Doernberg 1
Ravi Gupta 3
Joshua D Wallach 4
Objective To better understand the state of research on the effects of climate change on human health, including exposures, health conditions, populations, areas of the world studied, funding sources, and publication characteristics, with a focus on topics that are relevant for populations at risk.
Design Cross sectional study.
Data sources The National Institute of Environmental Health Sciences climate change and human health literature portal, a curated bibliographical database of global peer reviewed research and grey literature was searched. The database combines searches of multiple search engines including PubMed, Web of Science, and Google Scholar, and includes added-value expert tagging of climate change exposures and health impacts.
Eligibility criteria Inclusion criteria were peer reviewed, original research articles that investigated the health effects of climate change and were published in English from 2012 to 2021. After identification, a 10% random sample was selected to manually perform a detailed characterisation of research topics and publication information.
Results 10 325 original research articles were published between 2012 and 2021, and the number of articles increased by 23% annually. In a random sample of 1014 articles, several gaps were found in research topics that are particularly relevant to populations at risk, such as those in the global south (134 countries established through the United Nations Office for South-South Cooperation) (n=444; 43.8%), adults aged 65 years or older (n=195; 19.2%), and on topics related to human conflict and migration (n=25; 2.5%) and food and water quality and security (n=148; 14.6%). Additionally, fewer first authors were from the global south (n=349; 34.4%), which may partly explain why research focusing on these countries is disproportionally less.
Conclusions Although the body of research on the health effects of climate change has grown substantially over the past decade, including those with a focus on the global south, a disproportionate focus continues to be on countries in the global north and less at risk populations. Governments are the largest source of funding for such research, and governments, particularly in the global north, need to re-orient their climate and health research funding to support researchers in the global south and to be more inclusive of issues that are relevant to the global south.
What is already known on this topic
Climate change is one of the most pressing public health threats of the 21st century, contributing to more than 250 000 deaths each year
The environmental effects of climate change have increasingly important and varied implications for human health, disproportionately affecting vulnerable and oppressed populations, including people living in poverty
Research articles on the effects on human health of climate change increased eightfold between 2007 and 2019
What this study adds
The number of original research articles on human health and climate change published between 2012 and 2021 increased by 23% annually
Fewer research articles were relevant to at risk populations, eg, people in the global south and older adults, and were on topics related to human conflict, migration, and food and water quality and security
Articles studying the global south grew 41% annually, largely about China, but fewer articles compared the global south and global north, and a smaller number were published in high impact journals
How this study might affect research, practice, or policy
By 2100, 77% of the world's population is predicted to live in the global south
Funding for research on the effects of climate change on human health should therefore be directed towards investigators from the global south and topics relevant to populations more at risk
As populations living in the global south, particularly older adults and children, are less likely to have the resources to adapt to the effects of climate change more research is needed for these groups
- INTRODUCTION
Climate change is one of the most pressing public health threats of the 21st century, contributing to more than 250 000 deaths each year. 1 2 Global temperatures were 1.09°C warmer over 2011 to 2020 compared with 1850 to 1900, changing oceanic and atmospheric systems and contributing to rising sea levels, wild fires, flooding, droughts, and other extreme weather events. 3 The environmental effects of climate change have increasingly important and varied implications for human health through multiple pathways. Climate change has direct and indirect effects on morbidity and mortality, food and water insecurity, and social and economic challenges, such as financial stability and human displacement. 3 4 The type and severity of climate change effects vary by region, and countries in the global south that historically have contributed the least to anthropogenic climate change are often disproportionately affected. 1 4–7 Climate related stressors exacerbate existing inequalities within regions and disproportionately affect susceptible and oppressed populations, including people living in poverty. 8 9 Understanding the local economic, social, and environmental factors that affect how at risk a population is to climate change is critical to effectively and equitably address this threat worldwide. 10
Governments and researchers have been paying increasing attention to understanding the human health implications of climate change. 11 In 2013, the Intergovernmental Panel on Climate Change published a landmark report concluding that global warming is unequivocally the result of human actions. 12 In 2015, the same year that the Paris Agreement was adopted by 196 countries pledging to limit global warming to well below 2°C, preferably to 1.5°C, compared with pre-industrial levels, The Lancet created a commission on health and climate change that publishes a yearly report on the topic called the Lancet Countdown. 1 13 14 Beyond the Lancet Countdown, articles on the human health effects of climate change increased eightfold between 2007 and 2019. 1 According to a study that used machine learning to systematically map the existing literature on climate change and human health, the most commonly studied topics were heat related effects on health. 15 Major gaps in research were reported for non-physical health topics such as mental health, and the research was disproportionately produced by high income countries, consistent with prior studies. 1 3 6 16 The term global south includes the collection of postcolonial and low and middle income countries that united into the Group of 77 in 1964, and now includes 134 countries. Recognising the need to promote, coordinate, and support the interests of these countries, the United Nations established the UN Office for South-South Cooperation, which maintains the membership of this group and helps to coordinate cooperative goals and initiatives. In 2022, the UN Office for South-South Cooperation published a paper focusing on the impact of climate change on developing countries and discussed how the South-South Cooperation can help countries build climate resilience. 17 The global north is defined as all other countries.
While prior studies have characterised the topics studied in the literature on the health effects of climate change, publication characteristics and funding sources have not been comprehensively characterised. Understanding the sources of funding for literature that focuses on topics relevant to countries in the global south was also an unmet need. We conducted a cross-sectional study of a representative sample of original research on the human health effects of climate change published between 2012 and 2021 in order to better understand the state of research that has examined the effects of climate change on health. We characterised the climate change-related topics studied, including exposures, health conditions, populations, geographies studied, funding sources, and publishing characteristics, with a focus on differentiating what has been studied in the global south and global north. Details of the literature on climate change and human health and the topical emphases of prior studies can help to direct future research and policy efforts to topics and geographies with the greatest need.
Search strategy
We searched the National Institute of Environmental Health Sciences (NIEHS) climate change and human health literature portal, the largest curated bibliographical database of global peer-reviewed research and grey literature on the science of climate impact on human health, to identify peer-reviewed original research investigating the health effects of climate change published from 2012 to 2021. 18 19 At the time of our study, the portal's curation of 2022 articles was not complete, so we did not include articles published after 2021. The database combines searches of multiple search engines including PubMed, Web of Science, and Google Scholar and includes added-value expert tagging of climate change exposures and health impacts. This approach of identifying studies via screening records from an evidence hub, rather than relying on keyword searches in traditional bibliographical databases, provides a comprehensive and consistent source of climate change literature, as compared with independent keyword searches.
We filtered our search by publication year, limited to original research articles (defined as an article in a peer-reviewed journal containing original data) published in English. Search results for articles published between 2012 and 2019 were downloaded on 20 June 2021, and those for articles published in 2020 and 2021 were downloaded on 7 September 2023. After identifying all original research articles, we selected a 10% random sample to manually perform a detailed characterisation of research topics and publication information. Three investigators (VLB, HD, and MM) read the title and abstract to verify that the articles examined associations between climate change and health. Three investigators (VLB, HD, and MM) read the abstracts, and full text, if necessary, of each article in the sample to characterise the study characteristics. Each investigator validated a 5% random sample of the articles characterised by the other two investigators, with inter-rater reliability of 96.3%. Any uncertainties were clarified with a fourth investigator (JSR).
Study characteristics
For each article, we abstracted the climate change exposures, health effects, geographical locations, and populations studied. We also determined the publication year, journal impact factor, number of authors, location of the first author's affiliation, and funding sources. Climate change exposures were categorised into eight groups: general exposure; air pollution; extreme weather; food and water quality and security; human conflict and displacement; indoor environment; seasonality, temperature, and meteorological factors; and other. Health effects were categorised into 19 groups. These groups were based on the categories in the NIEHS portal. Articles were categorised as studying general health effects if they did not name a specific health effect but health was mentioned generally and the climate change exposure studied was one that clearly affects health (eg, water quality, food security, and heat waves). Geographical location was categorised as North America, South America, Europe, Asia, Africa, Australasia, Antarctica, global, or unspecified. Articles categorised as global were those in which specific geographies were not stated or global data were analysed in aggregate. We also characterised whether the article studied one or more countries in the global south, according to the United Nations' definition of the global south. 20 Articles that studied only countries in the global north were characterised as global north, and articles that studied only countries in the global south were also characterised as global south. If an article had a global focus, or if it studied countries in the global south and global north, we counted it in a third category of both global north and global south.
Population focuses were categorised as children, adults, older adults (aged 65 years or older), or not specified. Funding sources were categorised as government, non-profit, for-profit, academic, and not specified. The journal’s 2020 impact factor was obtained from Web of Science Journal Citation Reports, and categorised as 0-4.99, 5.00-9.99, or 10.00 and greater.
Statistical analyses
We used descriptive statistics to characterise the 10% sample. For categorical variables, we reported the percentage of articles within each category. For continuous variables, we reported the median and interquartile range. We also used the same methodology to characterise the subset of articles studying the global south. Within the random sample, we calculated the average annual percentage change in the number of articles for each variable if the total was greater than 20 articles. Data were analysed in Excel, version 16.54 (Microsoft).
Patient and public involvement
Patients and the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research. On publication, results will be widely disseminated through social media and will be sent directly to the National Institute of Environmental Health Sciences (NIEHS).
Number of articles and years published
Based on search results from the NIEHS portal on 20 July 2021 and 7 September 2023, we identified 10 325 original research articles published between 2012 and 2021: 297 (4.2%) articles were published in 2012, 187 (2.6%) in 2013, 845 (11.9%) in 2014, 831 (11.7%) in 2015, 1103 (15.6%) in 2016, 1196 (16.9%) in 2017, 1376 (19.4%) in 2018, 1247 (17.6%) in 2019, 1296 (13%) in 2020, and 1947 (19%) in 2021, representing a 23% compound annual growth ( figure 1 ). The largest year-to-year growth in the number of articles studied was from 2013 to 2014 (187 to 845 articles) and from 2020 to 2021 (1296 to 1947 articles).
Original research articles studying the association between climate change and health, 2012-21, in total (n=10 325) and in random 10% sample (n=1014)
In the random 10% sample of 1034 articles, 13 (1.3%) were excluded because they were not directly related to health or climate change. Seven (0.7%) were excluded because we were not able to access the full article. Of the remaining 1014 articles, 24 (2.4%) were published in 2012, 21 (2.1%) in 2013, 89 (8.8%) in 2014, 88 (8.7%) in 2015, 104 (10.3%) in 2016, 104 (10.3%) in 2017, 137 (13.5%) in 2018, 117 (11.5%) in 2019, 124 (12.2%) in 2020, and 195 (19.2%) in 2021. This represented a 26% compound annual growth in total number of articles published.
Climate change exposures studied
Among the 1014 articles, 697 (68.7%) studied seasonality, temperature, and meteorological factors, 318 (45.6%) of which studied the global south; 306 (30.2%) studied extreme weather, 119 (38.9%) of which studied the global south; and 148 (14.6%) studied food and water quality and security, 82 (55.4%) of which studied the global south ( table 1 ). The number of articles studying seasonality, temperature, and meteorological factors grew by 30% annually, in addition to the increase in number or articles studying extreme weather (28%) and air pollution (24%) ( figure 2 ).
(Top) Climate change exposures; (middle) health effects; and (bottom) geographies studied in original research articles studying the association between climate change and health, 2012-21
Health effects studied
Infectious disease was investigated by 288 (28.4%) of 1014 studies, of which 166 (57.6%) studied the global south; 244 (24.1%) articles studied general health, of which 107 (43.9%) studied the global south; 171 (16.9%) studied morbidity and mortality, of which 55 (32.2%) studied the global south; 82 (8.1%) studied economic effects, of which 41 (50.0%) studied the global south; and 53 (5.2%) studied mental health effects, of which 15 (28.3%) studied the global south ( table 1 ). The three most rapidly growing health effects studied were medical visits (34% yearly increase), temperature related (33% yearly increase), and mental health and wellbeing (22% yearly increase) ( figure 2 ).
Population geographical location studied
Of the 1014 sampled, Asia was the most commonly studied continent (n=358; 35.3%), followed by Europe (n=203; 20.0%), North America (n=192; 18.9%), Africa (n=101; 10.0%), Oceania (n=66; 6.5%), then South America (n=42; 4.1%). Fifty seven (5.6%) articles had a global focus, and 29 (2.9%) studies did not specify a geography ( table 1 ). The number of articles that studied Asia grew by 62% annually, those studying Africa grew by 30% annually, and those studying North America and South America both grew by 28% annually ( figure 2 ). In 2012, only four (16.7%) of the 24 articles studied the global south, whereas in 2021, 86 (44.1%) of 195 did, representing a 41% year-over-year growth ( figure 1 ). Almost half of the articles did not study any country in the global south (n=475; 46.8%). China was the most commonly studied country (n=138; 13.6%), followed by the United States (n=125; 12.3%), and Australia (n=51; 5.0%). Articles about China grew by 45% annually and made up 31.1% (138 of 444) of all articles that exclusively studied the global south.
Population demographics studied
Most articles did not specify an age related population or report the age distributions (eg, mean or median) (n=617; 60.8%), while 303 (29.9%) studied adult, 175 (17.3%) paediatric, and 195 (19.2%) older people populations ( table 1 ). Among articles with age related information, the populations in the global south that were studied were 128 (42.2%) adult, 94 (53.7%) paediatric, and 78 (40.0%) older people.
Author characteristics
The median number of authors per article was 5 (interquartile range 3-7). Of the 1014 articles, the most common first author's primary affiliation location was Asia (n=319; 31.5%), followed by Europe (n=276; 27.2%), North America (n=229; 22.6%), Oceania (n=80; 7.9%), Africa (n=42; 4.1%), and South America (n=21; 2.4%). The number of articles with the first author's primary affiliation in Asia grew by 60% annually, in Africa by 24% annually, and in Europe by 21% annually ( table 2 ). First authors with a primary affiliation in a global south country made up 34.4% (n=349) of articles, and those in a global north country 64.4% (n=653) of articles; author affiliations were missing for the remainder (n=12). The number of articles with a first author primary affiliation in a global south country grew by 43% annually. Of the 444 articles that exclusively studied the global south, 322 (72.5%) had a first author with their primary affiliation in the global south. Of the 653 articles with a first author's primary affiliation in the global north, 465 (71.2%) studied only countries in the global north, while 114 (17.5%) studied only countries in the global south. Most authors who had a primary affiliation in the global south studied countries in the global south (n=322; 92.3%), with the number of such articles growing by 40% annually.
Most of the 1014 articles were funded by government sources (n=618; 60.9%), while 225 (22.2%) were funded by academic sources, 83 (8.2%) by non-profit sources, and 10 (1.0%) by for-profit sources. Eighty seven (8.6%) articles stated they did not receive funding, while 193 (19.0%) did not specify whether or not they received funding for the work presented ( table 2 ).
Journal characteristics
In journals with an impact factor of less than five, 831 (82.0%) articles were published, of which 369 (44.4%) studied a country in the global south. Among the 161 (15.9%) articles that were published in journals with an impact factor of five to 9.99, 67 (41.6%) studied a country in the global south. Of the 23 (2.3%) articles published in journals with an impact factor of 10 and above, eight (34.8%) studied a country in the global south ( table 2 ). Articles in journals with an impact factor of five to 9.99 grew by 23% annually, and those with an impact factor of less than five grew by 28% annually.
Main findings
In this cross-sectional analysis of a random sample of original research articles that studied the effects on human health of climate change and published between 2012 and 2021, we found that the number of articles published increased by 23% annually. We found particularly steep increases from 2013 to 2014, and from 2020 to 2021. Despite this growth, we found several gaps in research topics that are relevant to more at risk populations, such as those in the global south and older people. Fewer first authors were from the global south, which may partly explain the disproportionally less research focusing on these countries, and may be linked to research funding sources and allocation.
The number of articles studying the global south grew by 41% annually, with a large portion of this driven by articles about China, which increased by 45% annually and made up almost a third of articles studying the global south. Despite this rapid growth, articles about the global south still constitute a smaller proportion of original research articles studying climate change and human health and a smaller percentage of them are published in high impact journals as compared with those studying the global north. However, by 2100, 77% of the world's population is predicted to live in the global south, where countries bear a disproportionate share of the effects of global warming. 21 22 In addition, the distribution of climate change exposures studied reflects a bias towards those that affect higher income countries. The two most studied topics—extreme weather and seasonality, and temperature and meteorology—had disproportionately fewer articles studying the global south. By contrast, articles studying human conflict and migration, all of which studied the global south, made up a small proportion of all articles. The impact of climate change on human conflict and migration is especially relevant for countries in the global south. Given that the number of climate refugees from Latin America, sub-Saharan Africa, and Southeast Asia alone is predicted to increase by 143 million people before 2050, research into this consequential climate change exposure and its potentially global effects is essential. 23 Similarly, food and water quality and security was also one of the least studied exposures, but most articles studied the global south, reflecting the disproportionate burden that these countries bear from this climate change exposure.
The distribution of illnesses studied also suggests disproportionate gaps in research that is most relevant to countries in the global south. The impact of climate change on infectious diseases and general health were the most commonly studied, while mental health and economic effects were studied by fewer articles. Evidence suggests that low income countries in the global south are the most affected by heat related reductions in labour capacity, with 2015 average estimated losses equivalent to 3.9-5.9% of gross domestic product in countries such as Indonesia, India, and Cambodia. 1 Moreover, financial stress is the strongest predictor of mental health issues after natural disasters, and people living in the global south are at increased risk of exposure to extreme weather events and poverty, and are less likely to have access to insurance to protect against damage from extreme weather events. 24
Few articles studied older people or children, both of whom are especially at risk populations. Fewer than half of the articles of older people were in the context of the global south, and about half of articles of paediatric populations studied the global south. Both children and older adults are especially susceptible to the effects of climate change, including extreme weather events, air quality, and infectious disease. 25 Moreover, low income older adults and children are less likely to have the resources to adapt to the effects of climate change. More research is needed to understand the unique needs of these at risk populations and to find geographically appropriate solutions.
Most articles' first authors were from countries in the global north, and less than a fifth of them studied global south countries. Additionally, articles studying global south countries were disproportionately published in journals with lower impact factors. Most articles received government funding, which may be less likely to fund studies that do not focus on their own geographical region. These findings may partially explain the disproportionate gap in quality and quantity of articles studying the global south observed. Moreover, health-related research bodies globally underfund and underemphasise research on climate change and health. 10 Greater funding of climate change and health research is needed, and particularly of researchers from countries in the global south and of studies that are relevant to the people that live in these locations, especially as the proportion of the world's population that live in the global south continues to grow. 26 This funding will require government, academic, non-profit, and for-profit sources in the global north to invest in and support investigators from the global south and their research.
Policy implications
Increasing the number and variety of research studies on the health effects of climate change may contribute to increased popular support for government engagement with climate change. Currently, only a small percentage of research on climate change focuses on its health effects. 1 In the annual United Nations General Assembly, engagement with the health effects of climate change is high in governments of island nations who face greater risks, particularly in the western Pacific region, while engagement is low among countries with the highest carbon dioxide emissions, including the United States and those in the European Union. 1 Increasing research on the effects of climate change, especially on topics relevant to the global south, may draw attention to and allow for increased investment in mitigating the health effects of climate change globally.
Limitations
There are several limitations to this study. Firstly, we manually characterised a random subset of all research articles. Although we did not consider all articles, the random 10% sample is a standard method and should be representative of the total population of research on climate change and human health. Secondly, when characterising the geographical location of the authors, we only considered the first author, so this may not reflect the full scope of affiliations of all co-authors. Thirdly, we did not include articles published before 2012, or those that were not published in English. This may have disproportionately affected our ability to capture articles written by investigators from the global south. However, the NIEHS portal offers a comprehensive resource that is most likely to identify the articles of greatest impact by researchers and those used by policy makers.
In this characterisation of original research on the health effects of climate change, we found that although the body of research has grown substantially over the past decade, including those with a focus on the global south, a disproportionate focus continues to be on countries in the global north and less at risk populations. By increasing funding for research on neglected topics, such as human conflict and displacement, economic effects, older populations, and countries in the global south, populations worldwide might be better equipped to address some of the most devastating potential effects of climate change. Governments are the largest source of funding for such research, and governments, particularly in the global north, need to re-orient their climate and health research funding to support researchers in the global south and to be more inclusive of issues that are relevant to the global south.
- Ethics approval
Not applicable.
- Publication history
- Rapid Responses
- Open access
- Published: 01 June 2018
Climate change and mental health: risks, impacts and priority actions
- Katie Hayes ORCID: orcid.org/0000-0001-8113-5828 1 ,
- G. Blashki 2 ,
- J. Wiseman 3 ,
- S. Burke 4 &
- L. Reifels 5
International Journal of Mental Health Systems volume 12 , Article number: 28 ( 2018 ) Cite this article
128k Accesses
259 Altmetric
Metrics details
This article provides an overview of the current and projected climate change risks and impacts to mental health and provides recommendations for priority actions to address the mental health consequences of climate change.
Discussion and conclusion
The authors argue the following three points: firstly, while attribution of mental health outcomes to specific climate change risks remains challenging, there are a number of opportunities available to advance the field of mental health and climate change with more empirical research in this domain; secondly, the risks and impacts of climate change on mental health are already rapidly accelerating, resulting in a number of direct, indirect, and overarching effects that disproportionally affect those who are most marginalized; and, thirdly, interventions to address climate change and mental health need to be coordinated and rooted in active hope in order to tackle the problem in a holistic manner. This discussion paper concludes with recommendations for priority actions to address the mental health consequences of climate change.
It is well understood that human health is threatened by the impacts of climate change [ 1 , 2 , 3 ]. In the 2017 Lancet Countdown on Climate Change and Health, authors state: “The human symptoms of climate change are unequivocal and potentially irreversible—affecting the health of populations around the world today” [ 4 ]. Climate change is no longer a looming threat but rather a destructive reality with dire predictions for the future. The World Health Organization (WHO) estimates an increase of 250,000 excess deaths per year between 2030 and 2050 due to the “well understood impacts of climate change” [ 5 ]. Impacts include heat-related morbidity and mortality, increases in vector-borne diseases (e.g. dengue fever, malaria), increased respiratory illness, and morbidity and mortality due to extreme weather events [ 6 , 7 ]. The lesser-known, and often overlooked, effects of climate change include the risks and impacts to mental health—the focus of this article.
Mental health refers not just to mental illness, mental problems, and mental disorders, but also includes states of mental wellness, emotional resilience and psychosocial wellbeing [ 8 , 9 , 10 , 11 ]. Psychosocial wellbeing is the interplay between social and psychological conditions that shape human welfare; a broad term which encompasses the states of being mentally healthy, experiencing mental problems, and mental illness [ 7 , 10 ].
Investigating the current state of evidence and knowledge about the climate change impacts to mental health, this article pays particular attention to the inequitable impacts of climate change on the mental health of marginalized and vulnerable populations. We argue the following three points: firstly, while attribution of mental health outcomes to specific climate change risks remains challenging, there are a number of opportunities available to advance the field of mental health and climate change with more empirical research in this domain; secondly, the risks and impacts of climate change on mental health are already rapidly accelerating, resulting in a number of direct, indirect, and overarching effects that disproportionally affect those who are most marginalized; and finally, interventions to address climate change and mental health need to be coordinated and rooted in active hope in order to tackle the problem in a holistic manner. This paper explores each of these facets and concludes with recommendations to enhance the state of knowledge and actions on climate change and mental health. Before diving into the topic area, a brief overview of climate change and health effects are noted in the sections below.
Climate change and health
An extensive body of research continues to strengthen knowledge about the impact of climate change on physical health, including for example, a rise in vector-borne, water and food-borne diseases; an increase in acute and chronic respiratory conditions (including asthma and allergies); and, heat-related and extreme weather-related morbidity and mortality [ 2 , 3 , 4 , 12 ]. Indirect health implications that are increasingly recognised in global reports on climate change and health include illness related to food and water safety, under-nutrition related to food insecurity, malignant melanoma from UV exposure, and chronic kidney disease from dehydration [ 4 ]. In late 2017, the Lancet released its first, full tracking report on climate change and health. In this report, there is an explicit request for more information on, and actions to address, the “often-unseen” impacts of climate change on human health, notably, the mental health consequences of climate change [ 4 ].
Climate change and mental health
The expanding research literature on climate change and mental health includes increasing evidence that extreme weather events—which are more frequent, intense, and complex under a changing climate—can trigger post-traumatic stress disorder (PTSD), major depressive disorder (MDD), anxiety, depression, complicated grief, survivor guilt, vicarious trauma, recovery fatigue, substance abuse, and suicidal ideation [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ]. Incremental climate changes, such as rising temperatures, rising sea levels, and episodic drought, can change natural landscapes, disrupt food and water resources, change agricultural conditions, change land use and habitation, weaken infrastructure and give rise to financial and relationship stress, increase risks of violence and aggression, and displacement of entire communities [ 4 , 18 , 23 , 27 , 28 ]. The overarching threats of a changing climate, can also incite despair and hopelessness as actions to address the ‘wicked problem’ of climate change seem intangible or insignificant in comparison to the scale and magnitude of the threats [ 29 ]. Paradoxically, these same disastrous circumstances may also inspire altruism, compassion, optimism, and foster a sense of meaning and personal growth (otherwise referred to as post-traumatic growth) as people band together to salvage, rebuild, and console amongst the chaos and loss of a changing climate [ 30 , 31 ].
Climate change and health inequity
It is well understood that climate change augments existing inequalities, rendering those most marginalized at greater peril to the health consequences of a changing climate [ 4 , 32 , 33 ]. In fact, the first key message from the Lancet’s Countdown on Climate Change and Health report emphasizes the disproportionate impact climate change has on the world’s most marginalized people and the consequential impacts this has on these populations if social and environment justice concerns are not addressed [ 4 ]. Watts et al. state: “By undermining the social and environmental determinants that underpin good health, climate change exacerbates social, economic, and demographic inequalities, with the impacts eventually felt by all populations” [ 4 ]. Those who are at greatest risk to the effects of climate change are those who are most marginalized based on socially and environmentally mediated factors, such as socioeconomic status, culture, gender, race, employment, and education [ 15 , 34 ]. Marginalized groups who tend to be the most affected by the mental and physical health implications of climate change are: Indigenous peoples, children, seniors, women, people with low-socioeconomic status, outdoor labourers, racialized people, immigrants, and people with pre-existing health conditions [ 2 , 3 , 7 , 13 , 22 , 23 , 33 , 35 , 36 ]. Importantly, these marginalized groups are not homogenous. People may experience intersections of marginalization based on a variety of the above social indicators.
Exploring the relationship between mental health and climate change
An updated overview of recent evidence on the mental health implications of climate change is timely given the ongoing, rapid expansion of research in the broad field of health and climate change as well as increasing public concern about climate change trends and risks. Footnote 1 Since 2007, media reports on climate change and health have increased by 78% and the academic literature on climate and health issues has tripled [ 4 ]. There is also increasing public and academic recognition of the extent to which rising global temperatures threaten planetary and human health [ 38 , 39 ]. While public awareness about the health implications of climate change continues to grow, the topic of mental health is frequently absent from this discourse. In some ways, this reflects the global discourse, where, in comparison to physical health, mental health in general has been neglected.
Globally, the prevalence of mental health issues is extremely high even without considering the added mental health consequences of a changing climate. Based on a 10-year systematic analysis of global burden of disease from 1990 to 2010, Murray et al. find that mental illness comprises 7.8% of the global burden of disease [ 40 ]. Mental and behavioural disorders also account for the greatest global burden of years lived with a disability (YLDs) [ 41 ]. Vigo et al. contend that these figures are actually much higher if co-morbidities related to mental illness are considered within burden of illness studies and if a more accurate definition of mental illness is used, a definition that includes health behaviours like self-harm and suicide [ 42 ]. The failure of global investment in mental health care to address the consequences and impacts of rapidly escalating levels of mental illness has been described as a “global tragedy” reflecting a long “legacy of the neglect and marginalization of mental health” [ 41 ]. Similarly, authors from the 2016 Lancet report on sustainable development and global mental health describe the state of mental health as the “most neglected of all human health conditions” and a “failure of humanity [ 43 ]. The inattention to mental health is of particular concern in the field of climate change and mental health given the evidence that psychological impacts from any form of disaster exceed physical injury by 40–1 [ 44 ], and that since 2000 the frequency of climate change-related weather disasters has increased by 46% [ 4 ]. Crucially, it is the most marginalized who are especially vulnerable to climate change’s impacts on mental health. As McMichael notes, climate change acts as a health “threat amplifier”, compounding existing social injustices [ 32 ]. There is, therefore, a strong case for continuing to explore and communicate research and policy learning about the relationship between climate change and mental health—especially as the topic area pertains to health equity.
Part 1. Exploring the challenges and opportunities of attribution
The lack of attention to the topic of climate change and mental health is often imputed to the challenges of attribution. Attribution in this case is the scientific association between greenhouse gas emissions and meteorological change on the one hand, and between climate change-related meteorological change and mental health effects on the other. There is now an increasingly strong body of literature which highlights the causal linkages between climate change and extreme weather events (see [ 45 , 46 , 47 , 48 , 49 , 50 ]). One of the key messages within this literature is that while we cannot say with certainty that any one specific extreme weather event is directly caused by climate change; we do know that because of climate change, extreme weather is more generally on the rise, making extreme weather events more frequent, intense, and complex. In other words, climate change therefore ‘loads the dice’ for more weather extremes.
Within the disaster mental health literature, the links between extreme weather events and mental health effects are well established (see for example [ 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 ]). However, many of these studies tend not to connect extreme weather to a changing climate—instead referring to extreme weather events as natural disasters rather than events linked to anthropogenic climate change. Studies within this domain tend to focus on mental health outcomes of specific hazard events (e.g. the 2004 Tsunami in Malaysia, Hurricane Katrina in 2005; Southern Alberta floods in 2013) positioning each hazard as an isolated incident unconnected to the wicked problem of climate change. The risk of overlooking or minimizing the role of climate change within these hazardous events is that this creates a reactive culture of emergency response that inhibits appropriate and effective adaptation planning and preparation for complex emergencies that a changing climate can create.
An additional concern is that much of the disaster mental health research has traditionally focussed on three distinct phases (the emergency and crisis stage, the post-impact stage, and the rehabilitation and recovery phase) [ 56 ], while little attention has been paid to psychosocial phenomena that can occur during the pre-disaster phase. Such phenomena include, for example, heightened anxiety levels, feelings of impending doom, hopelessness, and fatalism that can be triggered by approaching extreme events or associated weather warnings; and which may also be amplified due to the perceived risk of subacute, environmental changes like rising temperatures and episodic droughts [ 13 , 20 , 22 ].
Key challenges of attributing climate change to mental health
Attribution related to climate change and mental health can be challenging for four key reasons: firstly, there is a risk of pathologising common transitory distress responses to abnormal events and underdiagnosing mental health effects of a changing climate; secondly, there is a wide array of potential climate change and mental health outcomes related to a changing climate; thirdly, there is substantial scope with respect to the timing of the climate change effects on mental health, thus causal links become harder to determine; and finally, attribution related to climate change and mental health is not well understood because of the complex interaction between mental health and other social determinants of health.
There is a simultaneous risk of pathologising ‘normal’ responses to a changing climate and of underdiagnosing the real mental health effects of a changing climate. Pathologising ‘normal’ responses to disaster situations may result in a failure to differentiate between mild transitional distress or grief and more severe, persistent mental health problems. Both overinflating or underinflating mental health outcomes associated with climate change can lead to erroneous prevalence estimates and skewed assumptions about mental health service needs. A further consideration noted by Whaley in the aftermath of Hurricane Katrina is that in some cases medical professionals did not assess pre-existing mental health conditions and, therefore, attributed disaster trauma as a typical stress response, or alternatively diagnosed patients with stress response when in fact there were much larger mental health issues related to the effects of the Hurricane that went undiagnosed [ 60 ]. Crucially important to consider is that pre-existing mental health conditions or problems can be exacerbated or even triggered by changes in climate [ 9 , 61 ].
As noted earlier, mental health includes states of mental wellness as well as mental problems and disorders. With this in mind, the current application of tools to assess mental health have some limitations. Researchers tend to conceptualize mental health solely as mental illness and mental problems, administering surveys using validated instruments that assess mental health problems and issues like: generalized anxiety disorder (using the general anxiety disorder, GAD-2 questionnaire), PTSD (PCL-6 checklist), and psychological distress (via the general health questionnaire, GHQ-12) following an extreme weather event [ 52 , 53 , 62 , 63 , 64 , 65 , 66 ]. Few empirical studies that use these survey methods capture positive psychological consequences of extreme weather events, like feelings of compassion, altruism, sense of meaning, post-traumatic growth, or even increased acceptance of climate change and engagement with climate mitigation. This information can elucidate the complexity of mental health impacts from a changing climate and also help to understand any predisposing factors that may influence positive mental health outcomes and build psychosocial resilience.
Timing of psychosocial implications from climate related hazards poses another challenge. Scholars have discovered wide-ranging timeframes for the psychosocial impacts to manifest. Azuma et al. find that the incidence of psychological disorders (including PTSD) tended to be most significant within 6 months after a flood [ 62 ]. Kessler et al. conducted interviews with Hurricane Katrina survivors 5–8 months post-event and 1-year post-event, these authors found an increase in mental health disorders as time progressed [ 65 ]. For example, PTSD increased from 14.9% at 5–8 months to 20.9% after 1 year. Anderson et al. suggest that psychosocial impacts tend to peak within the first-year, post-extreme weather event [ 67 ]. Tunstall et al. on the other hand, found that residents who experience significant flooding self-report long-term psychosocial impacts (namely anxiety when it rains) from 2.5 to 5 years post-flooding [ 51 ]. A recent news article suggests that over 7000 people who experienced Hurricane Katrina in 2005 are still receiving mental health care for trauma associated with the Hurricane [ 68 ]. Noting that psychosocial health outcomes can have latent effects, or that these outcomes can occur as a result of sequelae, knowing how and when to study the climate change-related impacts and psychosocial outcomes becomes increasingly challenging—especially if the aim is to demonstrate the magnitude and attribution of effects.
Distilling the precise impact of climate change on mental health can be difficult to separate from other social determinants. As Watts et al. note, measuring the impacts of climate change on mental health is challenging not only because of attribution but also because of the “complicated nature of mental health, which embraces a diverse array of outcomes (e.g. anxiety and mood disorders), many of which co-occur and all of which vary with contexts and during lifetimes. Mental health impacts are often products of long and complex causal pathways, many of which can be traced back to distal but potent root causes, such as famine, war, and poverty, of which climate change is an accelerator” [ 4 ]. Mental health, like physical health, is shaped by social and ecological factors that can influence—and often amplify—other determinants of health, like a changing climate.
Opportunities of attributing mental health to climate change
It is important to locate climate change within the discourse on mental health because the frequency, intensity, duration, and complexity of climate change effects is on the rise and thus climate-related mental health outcomes are also increasing—adding to the already burgeoning burden of mental illness and mental problems globally. Acknowledging the mental health consequences of climate change helps the mental health community to discern and anticipate patterns of mental illness, like for example PTSD following extreme weather events. Also, an understanding of the unequal impacts of climate change on marginalized groups supports public health prevention strategies that seek to protect those most susceptible to mental illness and mental problems.
There is a risk that climate-related psychosocial consequences may become diluted in the high prevalence of mental health disorders globally; therefore, there is a need for additional research within the specific domain of climate change and mental health. If there is a better understanding of the linkages between climate change and mental health, there are more opportunities to understand and address climate change and mental health via actions rooted in climate change mitigation and adaptation that support psychosocial resilience. Specifically, the field requires more empirical research on the mental health consequences of climate change, especially as this research relates to marginalized communities and the risks and impacts associated with chronic climate change-related hazards and consequences (like sea-level rise, rising temperatures, and ecological degradation). To address the overarching interplay of social and environmental determinants of health that can magnify climate change-related risks on mental health, a health equity approach to this area of study is required. Secondly, there is a need to better understand climate change-related hazards within the context of mental health sequelae. Research in this area could help to explore the complexity of climate change and mental health attribution by recognizing the role of predisposing mental health conditions while also taking into account the perceived and actual risks and impacts related to a changing climate. This type of research can support a better understanding of the triggers and timing of climate change-related mental health effects as well as support policy and program development for mental health resources.
Attributing mental health outcomes to climate change also presents opportunities to assess, build, and strengthen mental health systems. In 2015, the World Health Organization (WHO) set forth the framework for building climate resilient health systems [ 69 ]. This framework provides guidance for health professionals to predict, prevent, and prepare for climate change-related shocks with the ultimate aim of protecting population-level health by increasing health systems’ capacity to cope, adapt, sustain, and strengthen in the wake of a changing climate. While the framework overlooks the intricacies of mental health systems—like the current and prevailing lack of mental health infrastructure, funding, and resourcing globally—it does provide the necessary guidance to build mental health systems resiliency. Articulating climate change as a determinant of mental health not only brings awareness of the broad consequences of climate change on health but also supports the enhancement of mental health systems.
In sum, the key benefits of understanding the linkages between climate change and mental health include: enhanced knowledge of patterns of illness; an added emphasis on the global call to action to reduce and address climate change risks and impacts; in-depth knowledge of the risks and impacts to marginalized communities; and, better planning for mental health response and mental health systems resiliency.
Part 2. Current risks and impacts of climate change on mental health
It is challenging for people to recognise changes in climate because these changes appear distant or abstract—especially because climate is often confused or lost in perceptions about weather or seasonal change [ 23 ]. The influential sociologist Anthony Giddens refers to this space and time distancing of the climate change problem as the Giddens Paradox [ 70 ]. The Giddens Paradox states that: “since the dangers posed by global warming aren’t tangible, immediate or visible in the course of day-to-day life, many will sit on their hands and do nothing of a concrete nature about them. Yet waiting until such dangers become visible and acute—in the shape of catastrophes that are irrefutably the result of climate change—before being stirred to serious action will be too late” (p. 2). Marshall contends that part of the time and space distancing of the climate change problem, and thus the reluctance to act, is reinforced by the Western political discourse on climate change as a future-facing problem that intentionally overlooks the centuries of industrialization, fossil fuel consumption, and land degradation that contribute to anthropogenic climate change [ 71 ]. Marshall calls for a reckoning with this discourse by noting:
“Climate change is a future problem. But it is also a past problem and a present problem. It is better thought of as a developing process of long-term deterioration, called, by some psychologists, a “creeping problem.” The lack of a definite beginning, end, or deadline requires that we create our own timeline. Not surprisingly, we do so in ways that remove the compulsion to act. We allow just enough history to make it seem familiar but not enough to create a responsibility for our past emissions. We make it just current enough to accept that we need to do something about it but put it just too far in the future to require immediate action” [ 71 ].
Noting the Giddens Paradox and the reckoning that Marshall asks us to have with the “creeping problem” of climate change, it becomes important to confront the current mental health consequences related to climate change that are happening now [ 70 , 71 ]. To do so, it is useful to explore the conceptual framework of climate change and mental health developed by Berry et al. [ 15 ]. These authors organize climate change-related hazards into three categories: acute (flooding, hurricanes, etc.), sub-acute (pervasive drought), and chronic (rising sea-level, increasing temperatures). These climate change-related hazards lead to a variety of direct, indirect, and overarching psychosocial consequences that are occurring now—disproportionately affecting those most marginalized.
Direct psychosocial consequences of climate change include trauma related to extreme weather events, like floods, hurricanes, wildfires, and heat waves [ 15 , 37 ]. Indirect mental health consequences of climate change occur through social, economic, and environmental disruptions (e.g. famine, civil conflict, displacement, and migration) related to a changing climate [ 15 , 37 ]. The overarching psychosocial consequences of climate change relate to the long-term emotional distress caused by awareness of the threats and impacts of climate change on the current and future wellbeing of the earth and its inhabitants. The multidimensional climate change and mental health pathway leads to a variety of unequal psychosocial consequences explored below.
Direct mental health consequences of climate change
There is now an extensive and rapidly expanding body of research exploring the current mental health consequences of climate change-related extreme weather events. Extreme heat events and humidity have been noted to increase hospital admissions for mood and behavioural disorders, including schizophrenia, mania, and neurotic disorders [ 72 , 73 ]. Scholars in the field note that heat-related mental health morbidity tends to occur most often in people with impaired thermoregulation, namely people with pre-existing mental health illness and problems, people taking prescription medications (specifically lithium, neuroleptic and anticholinergic drugs), and those with substance abuse (alcohol and drugs) problems [ 35 , 36 , 74 ]. Extreme heat is also linked with an increased risk of wildfires, which also directly impact mental health. Bryant et al. mapped the psychological outcomes of the Black Saturday bushfires in Victoria, Australia; in communities most at risk to the impacts of bushfires, these authors found incidences of PTSD, psychological distress, and depression related to the fires [ 75 ].
The direct mental health consequences related to flooding and hurricanes are also well documented (see [ 51 , 52 , 53 , 54 , 55 , 60 , 62 , 76 , 77 , 78 , 79 , 80 , 81 ]). In a study of 30 locations in England and Wales, Tunstall et al. conducted interview surveys with residents affected by flooding. They found that psychological impacts were more commonly reported than physical effects [ 51 ]. One study researching the psychosocial impacts following Hurricane Katrina estimates that 20–35% of survivors experienced some form of mental health issue following the disaster [ 60 ]. Galea et al. reported a 31.2% prevalence of anxiety-mood disorders amongst Hurricane Katrina survivors [ 81 ], while Rhodes and Chan found that nearly half (47.7%) of marginalised community members of New Orleans (mainly low-income, African American women) showed probable signs of PTSD after Hurricane Katrina [ 82 ].
While PTSD is often reported as one of the most severe mental health impacts related to acute climate change-related disasters, there have also been increasing reports of suicide and suicidal ideation following extreme weather events. Chand et al. note one Italian study that found higher rates of suicide in northern communities with greater climate variability [ 72 , 83 ]. Dodgen et al. highlight the risk of homicide-suicides after extreme weather events by noting the doubling of these incidents following Hurricane Andrew in 1992 in Miami-Dade County [ 74 ]. There is also observed evidence of increased suicidal thoughts (from 2.8 to 6.4%) and plans to commit suicide (from 1.0 to 2.5%) 18-months after an extreme weather event [ 74 ]. Notably, however, the overall evidence linking changing climate and suicide is still inconclusive. Studies on suicidality in natural disaster contexts, for example, vary considerably in study methodology and timeframes considered, with recent reviews indicating divergent trends in suicidality rates following exposure to extreme events, ranging from an initial decline, to neutral effects, all the way to a delayed increase in suicidality [ 84 ].
On a deeper level, the psychological responses of communities and individuals to disasters are complex and varied and do not necessarily simply result in more mental illnesses. Rebecca Solnit, in A Paradise Built in Hell , usefully describes the complicated psychosocial consequences that can arise after an extreme weather event as, “that sense of immersion in the moment and solidarity with others caused by the rupture in everyday life, an emotion graver than happiness but deeply positive. We don’t even have a language for this emotion, in which the wonderful comes wrapped in the terrible, joy in sorrow, courage in fear. We cannot welcome disaster, but we can value the responses, both practical and psychological” [ 85 ]. Exploring the complexity of psychological responses in the book, Climate change and human well - being , Weissbecker et al., discuss the full spectrum of psychosocial consequences of climate change-related events ranging from mental illness to more positive experiences like ‘Post Traumatic Growth’ (PTG), empathy, compassion, altruism, and emotional resilience [ 25 ].
Indirect mental health consequences of climate change
The indirect mental health consequences of climate change can occur as a result of damages to physical and social infrastructure, physical health effects, food and water shortages, conflict, and displacement from acute, subacute, and chronic climactic changes [ 15 ]. One of the most well-documented climate hazards that indirectly influences mental health is drought. Long-term droughts affect food and water supplies and can subsequently affect the economic and mental wellbeing of land-based workers, most often impacting those living in rural and remote communities [ 58 , 86 , 87 ]. In a quantitative analysis of drought and distress in Australia over a 7-year period, authors found that rural dwellers experience more distress due to the droughts than their urban counterparts [ 86 ]. In a systematic review of the literature, authors note the most prominent causal pathway linking drought and mental health is via the economic effects from land degradation [ 58 ]. These effects are most prominent amongst farmers whose economic livelihoods depend on environmental conditions. Exemplifying this, a 2008 study in New South Wales, Australia reports that nearly three quarters of farmers report stress related to persistent drought [ 88 ]. Some authors also suggest that income insecurity related to drought increases the risk for suicide among farmers [ 9 , 89 ].
Long-term drought has also been increasingly linked to conflict and forced migration, which can influence psychosocial outcomes like the propensity for stress, PTSD, anxiety, and trauma [ 90 ]. The Institute for Environment and Human Security of the United Nations University estimates that migration due to climate change may vary drastically, citing estimates of between 25 million to 1 billion by 2050, with 200 million as the most frequently cited estimate [ 91 ]. The rise in the number of ‘climate migrants’ has been identified as a significant risk by an increasing number of defence and security experts [ 92 , 93 ]. Gleick postulates that the civil conflict in Syria can be traced to the agricultural failures in 2006–2009 and the returning drought in 2011 [ 90 ]. In 2011, over 1.5 million Syrians moved from rural, agricultural areas to urban areas seeking refuge from the pervasive drought, failed agriculture, and lack of food and water [ 90 ]. Pervasive ecological degradation, poor policy response to water and food insecurity, and ongoing tensions between rural and urban community members, have arguably all contributed to civil unrest and ongoing conflict in Syria [ 90 ]. According to the United Nations, the number of displaced Syrians has reached over 5 million people in the past 5 years [ 94 ]. Migration from a war-torn country to a host country where culture, language, and lifestyle may be vastly different may also contribute to psychosocial malaise as displaced migrants can face stressors associated with xenophobia and racism from people in their new host country [ 90 ]. Conversely as Siriwardhana and Stewart note, displacement may also support psychosocial resilience by fostering hope and belonging for refugees in host countries where they feel welcomed, safe, and experience better living conditions [ 95 ].
At the community level the indirect mental health consequences of climate change are understudied. These consequences may include things like a diminishment in community cohesion, the loss of community identity, threats to a sense of continuity and sense of belonging as people are forced to move in and out of communities because of environmental stressors, and an undermining of cultural integrity if people have to leave their homelands [ 23 ]. Migration challenges the identity, sovereignty and heritage of people who have to leave their homelands. It also challenges the integrity and continuity of people’s traditional ways of life. Threats to community health also include an increased likelihood of criminal behaviour, violence and aggression as community members experience various stressors related to climate change [ 23 ].
Overarching psychosocial consequences of global climate change
Awareness of the looming threats and current risks and impacts of climate change presents challenges to emotional and social wellbeing [ 37 ]. Since early 2007, environmental philosopher Glenn Albrecht and colleagues have taken note of emotional distress related to the awareness of the overarching problem humans face as a result of global climate change [ 96 ]. Albrecht et al. suggest that this awareness contributes to ‘psychoterratic syndromes’. Psychoterratic syndromes include phenomena such as ‘ecoanxiety’, ‘ecoparalysis’, and ‘solastalgia’. ‘Ecoanxiety’ refers to the anxiety people face from constantly being surrounded by the wicked and threatening problems associated with a changing climate [ 96 ]. ‘Ecoparalysis’ refers to the complex feelings of not being able to take effective action to significantly mitigate climate change risks. ‘Solastalgia’ refers to “the distress and isolation caused by the gradual removal of solace from the present state of one’s home environment” [ 29 ]. The term ‘solastalgia’ is also commonly referred to throughout much of the literature on climate change and mental health to articulate the feelings associated with displacement following a climate change-related extreme weather event [ 17 ]. This new vocabulary provides the language to explore some of the broader mental health implications of escalating climate change risks.
For many people, climate change is experienced by way of vicarious threats or as an existential threat to civilisation [ 37 ]. People may experience vicarious threats when they receive weather warnings related to future disaster seasons or when they hear about environmental stressors experienced by people in other places. For many people, this is largely how climate change is experienced—not as a direct threat, but as a global threat, often distant in time and place, or as a threat to our very way of life. Qualitative research finds evidence of some people being deeply affected by feelings of loss, helplessness, and frustration as they engage with the problems of global climate change [ 97 ].
Part 3: Priority actions to address climate change and mental health
Acting on the health consequences of climate change requires actions rooted in both mitigation and adaptation at all levels—from global to local—and from all sectors and individuals. Climate change mitigation refers to overarching efforts to reduce greenhouse gas emissions and enhance carbon sinks to slow the speed, scale, and magnitude of climate change [ 98 ]. Key climate change mitigation priorities include reducing energy demand (through reduced consumption and increased energy efficiency); a swift and equitable transition from fossil fuels to renewable energy; reducing emissions from agriculture and forestry; and strengthening land-based emissions sequestration. Climate change adaptation refers to interventions that respond to the effects of climate change by adjusting, moderating, and coping with the risks and impacts of climate change [ 98 ]. Adaptation is ultimately affected by the capacity to adapt, which is the ability and willingness to respond to climate change mediated by individual and collective agency [ 99 ]. Adaptive capacity is determined by things like: governance, economics, infrastructure, technology, information and skills, institutions, and equity [ 100 ]. Examples of adaptation interventions that address climate change and health include: surveillance and monitoring of disease burdens related to climate change and health; education (e.g. public health promotion of the risks of vector-borne illness), and capacity building (e.g. psychological first-aid, and surge capacities at hospitals and health care facilities); preparing for extreme weather events; and re-locating entire communities to geographic areas where sea-level rise and frequent extreme weather events are less-likely to occur [ 7 ].
Within international approaches to combat climate change there is a significant focus not only on mitigation but also adaptation. From the Paris Accord to the Lancet Countdown on Climate Change, to the Planetary Manifesto and climate action marches—policy makers, academics, and the general population are taking steps to mitigate and adapt to the current threats and impacts to preserve a future for the next generation [ 4 , 39 ]. These actions, however, often fail to address the gap between stated goals of emissions reduction commitments and the speed of actions required to keep global warming well below 1.5–2 °C [ 101 ].
With a specific focus on mental health and climate change, there are a number of global programs in place that indirectly address the topic area—like for example measures to enhance and protect mental health in the Sustainable Development Goals 2016–2030 [ 102 ]; efforts by the Movement for Global Mental Health to increase the holistic conceptualization of health to incorporate mental health [ 103 ]; the Sendai Framework, a 15-year disaster risk reduction program [ 104 ]; and, the United Nations Human Settlement Program that promotes sustainable urban development [ 105 ]. There is a need, however, to harness health and mental health related synergies amongst these global agreements since none of these in and of themselves will likely be sufficient to address the future risks and impacts of climate change.
Coordinated, collaborative efforts to address the mental health implications of climate change not only require policy frameworks but also concrete actions on behalf of mental health practitioners. Such concrete actions may include: communicating about climate change and mental health in a way that helps people to see that it is relevant and salient to them; advocacy for greenhouse gas reductions in health care facilities and engagement in efforts to reduce the environmental footprint of the health care sector; and, engaging in adaptation measures like preparing for and responding to extreme events.
Psychological adaptation
Psychological adaptation requires a set of responses, it requires an acknowledgement of the grave threats posed by climate change and the profoundly consequential global crisis. It requires coping strategies to manage the feelings and thoughts that arise so that people can face up to, and come to terms with, these threats and consequences rather than avoiding the creeping problem of climate change. It also requires behavioural and psychological engagement, in which people change and adjust their behaviour and lifestyle in order to reduce the threat and protect themselves.
Active hope—something Macy and Johnstone champion—supports psychological adaptation. Active hope is required to move hopeful intentions from a passive state where waiting for someone else to take-on the task of addressing the climate change problem is replaced with an active process of climate change mitigation and adaptation behaviours [ 106 ]. The key point here is that hope alone cannot provide sufficient protection from the escalating risks of climate change. This active process occurs when the reality of the problem is acknowledged as is the magnitude of the problem, intentions to address the problem are set, and engaged actions take place. While these three steps may oversimplify the complexity of acting in the face of bureaucracy, climate denialism, or downright avoidance and ignorance of the magnitude of the problem area, these three steps are indeed the pivot points of transformation. These pivot points, however, need to be upheld by global political will and policy commitments that tackle the problem at the appropriate scale and speed. To do so, public awareness of the severity, magnitude and range of health impacts—current and projected—is required to pressure governments and communities to act now. Also, discernible interventions are needed to demonstrate a tangible path forward to respond to the risks and impacts we face in a changing climate. Examples of these types of interventions are explored below.
Adaptation measures
Adaptation measures that address the psychosocial impacts of climate change come in a variety of forms, i.e. policies, practices, behavioral interventions, community-based interventions, specific training, and pharmacotherapeutics. Some general approaches to address climate change-related mental health problems or illnesses include: primary care interventions, individual and group-based therapy, cognitive based interventions (including cognitive based therapy, cognitive restructuring, and, stress inoculation training), and crisis counselling [ 67 ]. More broadly, emotional resiliency may be sustained by engaging with art, literature, and spirituality. In addition to the above, the list below contains some specific priority adaptation mechanisms that ought to be considered to support population-level mental health in a changing climate:
Policy responses: improving access and funding to mental health care;
Surveillance and monitoring: administering epidemiological surveys after extreme weather events, and monitoring emergency department visits during heat waves and following extreme weather events;
Practice: the application of a stepped-care approach to mental health that is often used in disaster mental health to support different levels of interventions depending on the timing of the disaster and the level of distress (see [ 107 , 108 ]);
Preparation and response: climate change adaptation/resilience planning in the mental health system;
Community-based interventions: climate change resilience plans that address psychosocial wellbeing; and,
Special training for care providers and first responders: e.g. psychological first aid.
Other innovative approaches to addressing mental health and wellbeing in a changing climate writ large include experiencing and preserving nature. Koger et al. suggest that environmental preservation provides people with a sense of stewardship and personal investment that can help people overcome feelings of hopelessness, anxiety, and ecoparalysis [ 109 ]. Koger et al. suggest: “if people feel a deep connection to places, wilderness, and other species, then threats to these others are much more likely to be viewed as personal issues” [ 109 ]. Other research on the restorative benefits of natural environments and settings has found that biodiversity in natural environments is important for human health and wellbeing and has a particularly positive effect on mood, attention and cognition [ 110 ]. A common practice in Japan to reduce stress and anxiety is the practice of shinrin-yoku, otherwise referred to as forest bathing. In a study by Lee et al., authors found that forest bathing resulted in decreased cortisol levels, pulse rates, and negative feelings and significantly increased positive feelings [ 111 ]. Research on people’s interactions with nationally important ecosystems, like World Heritage Areas for example, highlights positive impacts including quality of life, a sense of place and belonging, self-identity, restoration and inspiration [ 112 ].
While there are a number of interventions to support psychosocial wellbeing within a changing climate, it is important to highlight that many of these interventions are still quite nascent and administered in ad hoc fashion, and these interventions are mainly accessible in developed countries. Sustainable mental health care in developed and developing nations is urgently needed as the realities of climate change become more and more apparent—especially for those most marginalized. Further, there are research needs in this domain where the efficacy and accessibility of mental health interventions-related to climate change are assessed.
Climate change affects mental health in a variety of direct, indirect, and overarching pathways—disproportionately affecting those most marginalized. The mental health implications of climate change can result in mental problems and illness as well as affirmative psychosocial outcomes. While the timing and triggers associated with climate change and mental health may vary, making it challenging to establish the manifold links between climate change and mental health, the opportunities of attributing mental health to climate change support climate mitigation as well as mental health action and psychosocial resiliency. Global commitments, like the Paris Accord, the SDGs, and the Sendai Framework are needed to help advance global mental health and climate action; however, coordination amongst these commitments is required—as are concrete actions on behalf of health practitioners—if the issue of mental health and a changing climate is to be efficiently and holistically addressed. Further, a reckoning with social, environmental, and climate injustice is needed if actions to address climate change and mental health are to be rooted in health equity. Transformative action—where inequities are addressed, active hope is demonstrated, and communities are mobilized—is the defining opportunity of the twenty-first century to address the climate change impacts on mental health.
This article builds on an earlier overview of mental health and climate change by Fritze et al. [ 37 ].
World Health Organization. WHO calls for urgent action to protect health from climate change—sign the call. 2015. http://www.who.int/globalchange/global-campaign/cop21/en/ . Accessed 11 Dec 2017.
Intergovernmental Panel on Climate Change. Summary for policymakers. In: Field, CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner G-K, Allen SK, Tignor M, and Midgley PM, editors. Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of working groups I and II of the intergovernmental panel on climate change. New York: Cambridge University Press; 2012.
Costello A, Abbas M, Allen A, Ball S, Bell S, Bellamy R, Lee M. Managing the health effects of climate change. Lancet. 2009;373(9676):1693–733.
Article PubMed Google Scholar
Watts N, Amann M, Ayeb-Karlsson S, Belesova K, Bouley T, Boykoff M, Byass P, Cai W, Campbell-Lendrum D, Chambers J, Cox PM, Daly M, Dasandi N, Davies M, Depledge M, Depoux A, Dominguez-Salas P, Drummond P, Ekins P, Flahault A, Frumkin H, Georgeson L, Ghanei M, Grace D, Graham H, Grojsman R, Haines A, Hamilton I, Hartinger S, Johnson A, Kelman I, Kiesewetter G, Kniveton D, Liang L, Lott M, Lowe R, Mace G, Odhiambo Sewe M, Maslin M, Mikhaylov S, Milner J, Latifi AM, Moradi-Lakeh M, Morrissey K, Murray K, Neville T, Nilsson M, Oreszczyn T, Owfi F, Pencheon D, Pye S, Rabbaniha M, Robinson E, Rocklöv J, Schütte S, Shumake-Guillemot J, Steinbach R, Tabatabaei M, Wheeler N, Wilkinson P, Gong P, Montgomery H, Costello A. The Lancet countdown on health and climate change: from 25 years of inaction to a global transformation for public health. Lancet. 2017. https://doi.org/10.1016/s0140-6736(17)32464-9 .
Article PubMed PubMed Central Google Scholar
Watts N, Adger WN, Agnolucci P, Blackstock J, Byass P, Cai W, Chaytor S, Colbourn T, Collins M, Cooper A, Cox PM, Depledge J, Drummond P, Ekins P, Galaz V, Grace D, Graham H, Grubb M, Haines A, Hamilton I, Hunter A, Jiang X, Li M, Kelman I, Liang L, Lott M, Lowe R, Luo Y, Mace G, Maslin M, Nilsson M, Oreszczyn T, Pye S, Quinn T, Svensdotter M, Venevsky S, Warner K, Xu B, Yang J, Yin Y, Yu C, Zhang Q, Gong P, Montgomery H, Costello A. Health and climate change: policy responses to protect public health. Lancet. 2015. https://doi.org/10.1016/s0140-6736(15)60854-6 .
United States Government Accountability Office. Climate change: information on potential economic effects could help guide federal efforts to reduce fiscal exposure. 2017. http://www.gao.gov/assets/690/687466.pdf . Accessed 12 Nov 2017.
Berry P, Clarke K-L, Parker S. Chapter 7: human health. In: Warren FJ, Lemmen DS, editors. Canada in a changing climate: sector perspectives on impacts and adaptation. Ottawa: Government of Canada; 2014. p. 191–232.
Google Scholar
Friedli L. Mental health, resilience and inequalities. World Health Organization. 2009. http://www.euro.who.int/__data/assets/pdf_file/0012/100821/E92227.pdf . Accessed 12 Dec 2017.
Butler CD, Bowles DC, McIver L, Page L. Mental health, cognition and the challenge of climate change. Clim Change Glob Health. 2014;26:251.
CAMH. Mental illness and addictions: facts and statistics. 2012. http://www.camh.ca/en/hospital/about_camh/newsroom/for_reporters/Pages/addictionmentalhealthstatistics.aspx . Accessed 6 Jan 2015.
World Health Organization. Mental health. 2017. http://www.who.int/mental_health/en/ . Accessed 10 Nov 2017.
McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. Lancet. 2006;367(9513):859–69.
Berry HL, Kelly BJ, Hanigan IC, Coates JH, McMichael AJ, Welsh JA, Kjellstrom T. Rural mental health impacts of climate change. Commissioned report for the Garnaut Climate Change Review. Canberra: The Australian National University; 2008.
Berry H. Pearl in the oyster: climate change as a mental health opportunity. Aust Psychiatry. 2009;17(6):453–6.
Article Google Scholar
Berry HL, Bowen K, Kjellstrom T. Climate change and mental health: a causal pathways framework. Int J Public Health. 2010;55(2):123–32.
Bourque F, Cunsolo Willox A. Climate change: the next challenge for public mental health? Int Rev Psychiatry. 2014;26(4):415–22.
Willox AC, Harper SL, Ford JD, Landman K, Houle K, Edge VL. “From this place and of this place:” climate change, sense of place, and health in Nunatsiavut, Canada. Soc Sci Med. 2012;75(3):538–47.
Willox AC, Harper SL, Edge VL, Landman K, Houle K, Ford JD. The land enriches the soul: on climatic and environmental change, affect, and emotional health and well-being in Rigolet, Nunatsiavut, Canada. Emotion Space Soc. 2013;6:14–24.
Willox AC, Harper SL, Ford JD, Edge VL, Landman K, Houle K, Blake S, Wolfrey C. Climate change and mental health: an exploratory case study from Rigolet, Nunatsiavut, Canada. Clim Change. 2013;121(2):255–70.
Willox AC, Stephenson E, Allen J, Bourque F, Drossos A, Elgarøy S, Kral MJ, Mauro I, Moses J, Pearce T, MacDonald JP. Examining relationships between climate change and mental health in the Circumpolar North. Reg Environ Change. 2015. https://doi.org/10.1007/s10113-014-0630-z .
Doherty TJ, Clayton S. The psychological impacts of global climate change. Am Psychol. 2011;66(4):265.
Clayton S, Manning C, Hodge C. Beyond storms & droughts: the psychological impacts of climate change. Washington, D.C: American Psychological Association and ecoAmerica; 2014.
Clayton S, Manning C, Krygsman K, Speiser M. Mental health and our changing climate: impacts, implications, and guidance. Washington, DC: American Psychological Association and ecoAmerica; 2017.
Coyle KJ, Van Susteren L. The psychological effects of global warming on the United States: and why the US mental health care system is not adequately prepared. National Wildlife Federation. 2012. http://www.climateaccess.org/sites/default/files/NWF_Psychological%20Effects.pdf . Accessed 12 Nov 2017.
Weissbecker I. Climate change and human well-being: global challenges and opportunities. Berlin: Springer; 2011.
Book Google Scholar
Swim J, Clayton S, Doherty T, Gifford R, Howard G, Reser J, Stern P, Weber E. Psychology and global climate change: addressing a multi-faceted phenomenon and set of challenges. A report by the American Psychological Association’s task force on the interface between psychology and global climate change. Washington: American Psychological Association; 2009.
Nurse J, Basher D, Bone A, Bird W. An ecological approach to promoting population mental health and well-being—a response to the challenge of climate change. Perspect Public Health. 2010;130(1):27–33.
Agnew R. Dire forecast: a theoretical model of the impact of climate change on crime. Theor Criminol. 2012;16(1):21–42.
Albrecht G. Chronic environmental change: emerging ‘psychoterratic’syndromes. In: Climate change and human well-being. New York: Springer; 2011. p. 43–56.
Ramsay T, Manderson L. Resilience, spirituality and posttraumatic growth: reshaping the effects of climate change. In: Weissbecker, editor. Climate change and human well-being. New York: Springer; 2011. p. 165–84.
Chapter Google Scholar
Edwards T, Wiseman J. Climate change, resilience and transformation: challenges and opportunities for local communities. In: Weissbecker, editor. Climate change and human well-being. New York: Springer; 2011. p. 185–200.
McMichael A. climate change and the health of nations: famines, fevers, and the fate of populations. Oxford: Oxford University Press; 2017.
Trombley J, Chalupka S, Anderko L. Climate change and mental health. AJN Am J Nurs. 2017;117(4):44–52.
Gamble JL, Balbus J, Berger M, Bouye K, Campbell V, Chief K, Conlon K, Crimmins A, Flanagan B, Gonzalez-Maddux C, Hallisey ECh. 9: populations of concern. Washington, DC: US Global Change Research Program; 2016.
Cusack L, de Crespigny C, Athanasos P. Heatwaves and their impact on people with alcohol, drug and mental health conditions: a discussion paper on clinical practice considerations. J Adv Nurs. 2011;67(4):915–22.
Page LA, Hajat S, Kovats RS, Howard LM. Temperature-related deaths in people with psychosis, dementia and substance misuse. Br J Psychiatry. 2012;200(6):485–90.
Fritze JG, Blashki GA, Burke S, Wiseman J. Hope, despair and transformation: climate change and the promotion of mental health and wellbeing. Int J Ment Health Syst. 2008;2(1):13.
Hancock T, Spady DW, Soskolne CL, editors. Global change and public health: addressing the ecological determinants of health—the report in brief. 2015. http://www.cpha.ca/uploads/policy/edh-brief.pdf . Accessed 7 Nov 2017.
Horton R, Beaglehole R, Bonita R, Raeburn J, McKee M, Wall S. From public to planetary health: a manifesto. Lancet. 2014;383(9920):847.
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2013;380(9859):2197–223.
Becker AE, Kleinman A. Mental health and the global agenda. N Engl J Med. 2013;369(1):66–73.
Article PubMed CAS Google Scholar
Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3(2):171–8.
Patel V, Saxena S, Frankish H, Boyce N. Sustainable development and global mental health—a Lancet Commission. Lancet. 2016;387(10024):1143–5.
Links J. Predicting community resilience and recovery after a disaster. CDC. 2017. https://blogs.cdc.gov/publichealthmatters/2017/08/predicting-community-resilience-and-recovery-after-a-disaster/ . Accessed 1 Sept 2017.
National Academies of Sciences. Engineering, and medicine. Attribution of extreme weather events in the context of climate change. Washington, DC: National Academies Press; 2016.
Stott PA, Stone DA, Allen MR. Human contribution to the European heatwave of 2003. Nature. 2004;432(7017):610–4.
Stone DA, Allen MR. The end-to-end attribution problem: from emissions to impacts. Clim Change. 2005;71(3):303–18.
Easterling DR, Kunkel KE, Wehner MF, Sun L. Detection and attribution of climate extremes in the observed record. Weather Clim Extremes. 2016;11:17–27.
Pall P, Aina T, Stone DA, Stott PA, Nozawa T, Hilberts AG, Lohmann D, Allen MR. Anthropogenic greenhouse gas contribution to flood risk in England and Wales in autumn 2000. Nature. 2011;470(7334):382–5.
Otto FEL, Massey N, van Oldenborgh GJ, Jones RG, Allen MR. Reconciling two approaches to attribution of the 2010 Russian heat wave. Geophys Res Lett. 2012;39:L04702. https://doi.org/10.1029/2011GL050422 .
Tunstall S, Tapsell S, Green C, Floyd P, George C. The health effects of flooding: social research results from England and Wales. J Water Health. 2006;4(3):365–80.
Waite TD, Chaintarli K, Beck CR, Bone A, Amlôt R, Kovats S, Reacher M, Armstrong B, Leonardi G, Rubin GJ, Oliver I. The English national cohort study of flooding and health: cross-sectional analysis of mental health outcomes at year one. BMC Public Health. 2017;17(1):129.
Alderman K, Turner LR, Tong S. Assessment of the health impacts of the 2011 summer floods in Brisbane. Disaster Med Public Health Preparedness. 2013;7(4):380–6.
Fernandez A, Black J, Jones M, Wilson L, Salvador-Carulla L, Astell-Burt T, Black D. Flooding and mental health: a systematic mapping review. PLoS One. 2015;10(4):e0119929. https://doi.org/10.1371/journal.pone.0119929 .
Article PubMed PubMed Central CAS Google Scholar
Stanke C, Murray V, Amlôt R, Nurse J, Williams R. The effects of flooding on mental health: outcomes and recommendations from a review of the literature. PLoS Curr. 2012;4:e4f9f1fa9c3cae. https://doi.org/10.1371/4f9f1fa9c3cae .
Chakrabhand S, Panyayong B, Sirivech P. Mental health and psychosocial support after the tsunami in Thailand. Int Rev Psychiatry. 2006;18(6):599–605.
Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: part I. An empirical review of the empirical literature, 1981–2001. Psychiatry. 2002;65(3):207–39.
Vins H, Bell J, Saha S, Hess JJ. The mental health outcomes of drought: a systematic review and causal process diagram. Int J Environ Res Public Health. 2015;12(10):13251–75.
Sahni V, Scott AN, Beliveau M, Varughese M, Dover DC, Talbot J. Public health surveillance response following the southern Alberta floods, 2013. Can J Public Health. 2016;107(2):142–8.
Whaley AL. Trauma among survivors of Hurricane Katrina: considerations and recommendations for mental health care. J Loss Trauma. 2009;14(6):459–76.
North CS, Pfefferbaum B. Mental health response to community disasters: a systematic review. JAMA. 2013;310(5):507–18.
Azuma K, Ikeda K, Kagi N, Yanagi U, Hasegawa K, Osawa H. Effects of water-damaged homes after flooding: health status of the residents and the environmental risk factors. Int J Environ Health Res. 2014;24(2):158–75.
Crabtree A. Climate change and mental health following flood disasters in developing countries, a review of the epidemiological literature: what do we know, what is being recommended? Aust J Disaster Trauma Stud. 2012;12(1):21–30.
Eisenman D, McCaffrey S, Donatello I, Marshal G. An ecosystems and vulnerable populations perspective on solastalgia and psychological distress after a wildfire. EcoHealth. 2015;12(4):602–10.
Kessler RC, Galea S, Gruber MJ, Sampson NA, Ursano RJ, Wessely S. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374–84.
Greene G, Paranjothy S, Palmer SR. Resilience and vulnerability to the psychological harm from flooding: the role of social cohesion. Am J Public Health. 2015. https://doi.org/10.2105/AJPH.2015.302709 .
Anderson H, Brown C, Cameron LL, Christenson M, Conlon KC, Dorevitch S. Climate and health intervention assessment: evidence on public health interventions to prevent the negative health effects of climate change. Climate and health technical report series. BRACE Midwest and Southeast Community of Practice. Climate and Health Program, Centers for Disease Control and Prevention. 2017.
Vestal C. ‘Katrina brain’: the invisible long-term toll of megastorms. POLITICO. 2017. http://www.politico.com/agenda/story/2017/10/12/psychological-toll-natural-disasters-000547 . Accessed 12 Oct 2017.
World Health Organization. Operational framework for building climate resilient health systems. 2015. http://www.who.int/globalchange/publications/building-climate-resilient-health-systems/en/ . Accessed 20 Sept 2017.
Giddens A. The politics of climate change. UK: Cambridge; 2009.
Marshall G. Don’t even think about it: why our brains are wired to ignore climate change. London: Bloomsbury Publishing; 2015.
Chand PK, Murthy P. Climate change and mental health. Reg Health Forum. 2008;12(1):43–8.
Wang H, Horton R. Tackling climate change: the greatest opportunity for global health. Lancet. 2015;386(10006):1798–9.
Dodgen D, Donato D, Kelly N, La Greca A, Morganstein J, Reser J, Ruzek J, Schweitzer S, Shimamoto MM, Tart KT, Ursano RCh. 8: mental health and well-being. Washington, DC: US Global Change Research Program; 2016.
Bryant R, Waters E, Gibbs L, Gallagher C, Pattison P, Lusher D, MacDougall C, Harms L, Block K, Snowdon E, Sinnott V, Ireton G, Richardson J, Forbes D. Psychological outcomes following the Victorian Black Saturday bushfires. Aust NZ J Psychiatry. 1014;48(7):634–43.
Burton H, Rabito F, Danielson L, Takaro TK. Health effects of flooding in Canada: a 2015 review and description of gaps in research. Can Water Resourc J/Revue canadienne des ressources hydriques. 2016;41(1–2):238–49.
Neria Y, Shultz JM. Mental health effects of Hurricane Sandy: characteristics, potential aftermath, and response. JAMA. 2012;308(24):2571–2.
Schmeltz MT, González SK, Fuentes L, Kwan A, Ortega-Williams A, Cowan LP. Lessons from hurricane sandy: a community response in Brooklyn, New York. J Urban Health. 2013;90(5):799–809.
Osofsky JD, Osofsky HJ, Kronenberg M, Hansel TC. The aftermath of Hurricane Katrina: mental health considerations and lessons learned, chapter 10. In: Helping families and communities recover from disaster. Washington, D.C: American Psychological Association; 2010. p. 241–63.
Haskett ME, Scott SS, Nears K, Grimmett MA. Lessons from Katrina: disaster mental health service in the Gulf Coast region. Prof Psychol. 2008;39(1):93.
Galea S, Brewin CR, Gruber M, Jones RT, King DW, King LA, McNally RJ, Ursano RJ, Petukhova M, Kessler RC. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12):1427–34.
Rhodes J, Chan C, Paxson C, Rouse CE, Waters M, Fussell E. The impact of hurricane Katrina on the mental and physical health of low-income parents in New Orleans. Am J Orthopsychiatry. 2010;80(2):237.
Preti A, Miotto P. Seasonality in suicides: the influence of suicide method, gender and age on suicide distribution in Italy. Psychiatry Res. 1998;81(2):219–31.
Reifels L, Spittal MJ, Dückers M, Mills KL, Pirkis J. Suicidality risk and (repeat) disaster exposure: findings from a nationally representative population survey. Psychiatry Interpers Biolog Process. 2018. https://doi.org/10.1080/00332747.2017.1385049 .
Solnit R. A paradise built in hell: the extraordinary communities that arise in disaster. New York: Penguin; 2010.
OBrien LV, Berry HL, Coleman C, Hanigan IC. Drought as a mental health exposure. Environ Res. 2014;131:181–7.
Yusa A, Berry P, Cheng J, Ogden N, Bonsal B, Stewart R, Waldick R. Climate change, drought and human health in Canada. Int J Environ Res Public Health. 2015;12(7):8359–412.
Stain HJ, Kelly B, Lewin TJ, Higginbotham N, Beard JR, Hourihan F. Social networks and mental health among a farming population. Soc Psychiatry Psychiatr Epidemiol. 2008;43(10):843.
Ellis NR, Albrecht GA. Climate change threats to family farmers’ sense of place and mental wellbeing: a case study from the Western Australian wheatbelt. Soc Sci Med. 2017;175:161–8.
Gleick PH. Water, drought, climate change, and conflict in Syria. Weather Clim Soc. 2014;6(3):331–40.
IEHS. 5 Facts on climate migrants. United Nations University. 2015. https://ehs.unu.edu/blog/5-facts/5-facts-on-climate-migrants.html . Accessed 12 Dec 2017.
Department of Defense. National security implications of climate-related risks and a changing climate. 2015. http://archive.defense.gov/pubs/150724-congressional-report-on-national-implications-of-climate-change.pdf?source=govdelivery . Accessed 14 Dec 2017.
Gemenne F, Barnett J, Adger WN, Dabelko GD. Climate and security: evidence, emerging risks, and a new agenda. Clim Change. 2014;123(1):1–9.
Aljazeera. UN: number of Syrian refugees passes five million. 2017. http://www.aljazeera.com/news/2017/03/number-syrian-refugees-passes-million-170330132040023.html . Accessed 14 Dec 2017.
Siriwardhana C, Stewart R. Forced migration and mental health: prolonged internal displacement, return migration and resilience. Int Health. 2013;5(1):19–23.
Albrecht G, Sartore GM, Connor L, Higginbotham N, Freeman S, Kelly B, Stain H, Tonna A, Pollard G. Solastalgia: the distress caused by environmental change. Aust Psychiatry. 2007;15(Suppl 1):S95–8. https://doi.org/10.1080/1039856070170128 .
Moser SC. Navigating the political and emotional terrain of adaptation: community engagement when climate change comes home. Successful adaptation to climate change: linking science and policy in a rapidly changing world. New York: Routledge; 2013. p. 289–305.
Allwood JM, Bosetti V, Dubash NK, Gómez-Echeverri L, von Stechow C. Glossary. In: Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann B, Savolainen J, Schlömer S, von Stechow C, Zwickel T, Minx JC, editors. Climate change 2014: mitigation of climate change Contribution of working group III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2014.
Brown K, Westaway E. Agency, capacity, and resilience to environmental change: lessons from human development, well-being, and disasters. Ann Rev Environ Resourc. 2011;36:321–42. https://doi.org/10.1146/annurev-environ-052610-092905 .
Séguin J, Berry P, Bouchet V, Clarke KL, Furgal C, Environmental I, MacIver D. Human health in a changing climate: a Canadian assessment of vulnerabilities and adaptive capacity. In: Human health in a changing climate. 2008.
UN Environment. Emissions gap report. UNEP Synthesis Report. 2017. https://www.unenvironment.org/resources/emissions-gap-report . Accessed 23 Feb 2018.
United Nations. Sustainable development goals. 2017. https://sustainabledevelopment.un.org/sdgs . Accessed 2 Jan 2018.
Movement for Global Mental Health. N.d. http://www.globalmentalhealth.org . Accessed 2 Jan 2018.
United Nations Office for Disaster Risk Reduction. Sendai framework for disaster risk reduction. 2015. http://www.unisdr.org/we/coordinate/sendai-framework . Accessed 2 Jan 2018.
United Nations. United Nations human settlements programme (UN-HABITAT). 2015. https://www.un-ngls.org/index.php/engage-with-the-un/un-civil-society-contact-points/144-united-nations-human-settlements-programme-un-habitat . Accessed 2 Jan 2018.
Macy J, Johnstone C. Active hope: how to face the mess we’re in without going crazy. California: New World Library; 2012.
Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Br J Psychiatry. 2005;186(1):11–7.
Twomey C, Byrne M. A stepped care approach to mental health. Forum. 2012;29(11):31–2.
Koger SM, Leslie KE, Hayes ED. Climate change: psychological solutions and strategies for change. Ecopsychology. 2011;3(4):227–35.
Maas J, Verheij R, Groenewegen P, de Vries S, Spreeuwenberg P. Green space, urbanity and health: how strong is the relationship? J Epidemiol Community Health. 2006;60:587–92.
Lee J, Park BJ, Tsunetsugu Y, Ohira T, Kagawa T, Miyazaki Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health. 2011;125(2):93–100.
Bentrupperbäumer JM, Reser JP. Encountering a World Heritage landscape: community and visitor perspectives and experiences. In: Stork N, Turton S, editors. Living in a dynamic tropical forest landscape: lessons from Australia. Oxford: Blackwell Publishing; 2008. p. 387–97.
Download references
Authors’ contributions
GB conceived of this paper and developed the paper outline. KH undertook the literature review, developed the paper and incorporated co-author contributions. GB, JW, SB, and LR contributed generally to successive iterations of the paper. All authors read and approved the final manuscript.
Acknowledgements
Thank you to the anonymous reviewers for their valuable feedback.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Ethics approval and consent to participate.
There are no funding arrangements to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Katie Hayes
Nossal Institute for Global Health, University of Melbourne, Carlton, VIC, Australia
Melbourne Sustainable Society Institute, University of Melbourne, Carlton, VIC, Australia
Australian Psychological Society, Level 11, 257 Collins St, Melbourne, VIC, Australia
Centre for Mental Health, Melbourne School of Population and Global Health, The University of Melbourne, Carlton, VIC, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Katie Hayes .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Hayes, K., Blashki, G., Wiseman, J. et al. Climate change and mental health: risks, impacts and priority actions. Int J Ment Health Syst 12 , 28 (2018). https://doi.org/10.1186/s13033-018-0210-6
Download citation
Received : 27 February 2018
Accepted : 27 May 2018
Published : 01 June 2018
DOI : https://doi.org/10.1186/s13033-018-0210-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Climate change
- Mental health
- Attribution
International Journal of Mental Health Systems
ISSN: 1752-4458
- Submission enquiries: [email protected]
- Introduction
- Conclusions
- Article Information
BMI indicates body mass index; SES, socioeconomic status.
a Variables smoking status, SES, drinking pattern, former drinker bias only, occasional drinker bias, median age, and gender were removed.
b Variables race, diet, exercise, BMI, country, follow-up year, publication year, and unhealthy people exclusion were removed.
eAppendix. Methodology of Meta-analysis on All-Cause Mortality and Alcohol Consumption
eReferences
eFigure 1. Flowchart of Systematic Search Process for Studies of Alcohol Consumption and Risk of All-Cause Mortality
eTable 1. Newly Included 20 Studies (194 Risk Estimates) of All-Cause Mortality and Consumption in 2015 to 2022
eFigure 2. Funnel Plot of Log-Relative Risk (In(RR)) of All-Cause Mortality Due to Alcohol Consumption Against Inverse of Standard Error of In(RR)
eFigure 3. Relative Risk (95% CI) of All-Cause Mortality Due to Any Alcohol Consumption Without Any Adjustment for Characteristics of New Studies Published between 2015 and 2022
eFigure 4. Unadjusted, Partially Adjusted, and Fully Adjusted Relative Risk (RR) of All-Cause Mortality for Drinkers (vs Nondrinkers), 1980 to 2022
eTable 2. Statistical Analysis of Unadjusted Mean Relative Risk (RR) of All-Cause Mortality for Different Categories of Drinkers for Testing Publication Bias and Heterogeneity of RR Estimates From Included Studies
eTable 3. Mean Relative Risk (RR) Estimates of All-Cause Mortality Due to Alcohol Consumption up to 2022 for Subgroups (Cohorts Recruited 50 Years of Age or Younger and Followed up to 60 Years of Age)
Data Sharing Statement
- Errors in Figure and Supplement JAMA Network Open Correction May 9, 2023
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Digital Health
- Drug Development
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Sexual Health
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Zhao J , Stockwell T , Naimi T , Churchill S , Clay J , Sherk A. Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses . JAMA Netw Open. 2023;6(3):e236185. doi:10.1001/jamanetworkopen.2023.6185
Manage citations:
© 2024
- Permissions
Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses
- 1 Canadian Institute for Substance Use Research, University of Victoria, Victoria, British Columbia, Canada
- 2 Department of Psychology, University of Portsmouth, Portsmouth, Hampshire, United Kingdom
- Correction Errors in Figure and Supplement JAMA Network Open
Question What is the association between mean daily alcohol intake and all-cause mortality?
Findings This systematic review and meta-analysis of 107 cohort studies involving more than 4.8 million participants found no significant reductions in risk of all-cause mortality for drinkers who drank less than 25 g of ethanol per day (about 2 Canadian standard drinks compared with lifetime nondrinkers) after adjustment for key study characteristics such as median age and sex of study cohorts. There was a significantly increased risk of all-cause mortality among female drinkers who drank 25 or more grams per day and among male drinkers who drank 45 or more grams per day.
Meaning Low-volume alcohol drinking was not associated with protection against death from all causes.
Importance A previous meta-analysis of the association between alcohol use and all-cause mortality found no statistically significant reductions in mortality risk at low levels of consumption compared with lifetime nondrinkers. However, the risk estimates may have been affected by the number and quality of studies then available, especially those for women and younger cohorts.
Objective To investigate the association between alcohol use and all-cause mortality, and how sources of bias may change results.
Data Sources A systematic search of PubMed and Web of Science was performed to identify studies published between January 1980 and July 2021.
Study Selection Cohort studies were identified by systematic review to facilitate comparisons of studies with and without some degree of controls for biases affecting distinctions between abstainers and drinkers. The review identified 107 studies of alcohol use and all-cause mortality published from 1980 to July 2021.
Data Extraction and Synthesis Mixed linear regression models were used to model relative risks, first pooled for all studies and then stratified by cohort median age (<56 vs ≥56 years) and sex (male vs female). Data were analyzed from September 2021 to August 2022.
Main Outcomes and Measures Relative risk estimates for the association between mean daily alcohol intake and all-cause mortality.
Results There were 724 risk estimates of all-cause mortality due to alcohol intake from the 107 cohort studies (4 838 825 participants and 425 564 deaths available) for the analysis. In models adjusting for potential confounding effects of sampling variation, former drinker bias, and other prespecified study-level quality criteria, the meta-analysis of all 107 included studies found no significantly reduced risk of all-cause mortality among occasional (>0 to <1.3 g of ethanol per day; relative risk [RR], 0.96; 95% CI, 0.86-1.06; P = .41) or low-volume drinkers (1.3-24.0 g per day; RR, 0.93; P = .07) compared with lifetime nondrinkers. In the fully adjusted model, there was a nonsignificantly increased risk of all-cause mortality among drinkers who drank 25 to 44 g per day (RR, 1.05; P = .28) and significantly increased risk for drinkers who drank 45 to 64 and 65 or more grams per day (RR, 1.19 and 1.35; P < .001). There were significantly larger risks of mortality among female drinkers compared with female lifetime nondrinkers (RR, 1.22; P = .03).
Conclusions and Relevance In this updated systematic review and meta-analysis, daily low or moderate alcohol intake was not significantly associated with all-cause mortality risk, while increased risk was evident at higher consumption levels, starting at lower levels for women than men.
The proposition that low-dose alcohol use protects against all-cause mortality in general populations continues to be controversial. 1 Observational studies tend to show that people classified as “moderate drinkers” have longer life expectancy and are less likely to die from heart disease than those classified as abstainers. 2 Systematic reviews and meta-analyses of this literature 3 confirm J-shaped risk curves (protective associations at low doses with increasing risk at higher doses). However, mounting evidence suggests these associations might be due to systematic biases that affect many studies. For example, light and moderate drinkers are systematically healthier than current abstainers on a range of health indicators unlikely to be associated with alcohol use eg, dental hygiene, exercise routines, diet, weight, income 4 ; lifetime abstainers may be systematically biased toward poorer health 5 ; studies fail to control for biases in the abstainer reference group, in particular failing to remove “sick quitters” or former drinkers, many of whom cut down or stop for health reasons 2 ; and most studies have nonrepresentative samples leading to an overrepresentation of older White men. Adjustment of cohort samples to make them more representative has been shown to eliminate apparent protective associations. 6 Mendelian randomization studies that control for the confounding effects of sociodemographic and environmental factors find no evidence of cardioprotection. 7
We published 2 previous systematic reviews and meta-analyses that investigated these hypotheses. The first of these focused on all-cause mortality, 8 finding negligible reductions in mortality risk with low-volume alcohol use when study-level controls were introduced for potential bias and confounding, such as the widespread practice of misclassifying former drinkers and/or current occasional drinkers as abstainers (ie, not restricting reference groups to lifetime abstainers). 8 Our alcohol and coronary heart disease (CHD) mortality meta-analysis of 45 cohort studies 9 found that CHD mortality risk differed widely by age ranges and sex of study populations. In particular, young cohorts followed up to old age did not show significant cardio-protection for low-volume use. Cardio-protection was only apparent among older cohorts that are more exposed to lifetime selection biases (ie, increasing numbers of “sick-quitters” in the abstainer reference groups and the disproportionate elimination of drinkers from the study sample who had died or were unwell).
The present study updates our earlier systematic review and meta-analysis for all-cause mortality and alcohol use, 8 including studies published up to July 2021 (ie, 6.5 years of additional publications). The study also investigated the risk of all-cause mortality for alcohol consumption according to (1) median ages of the study populations (younger than 56 years or 56 years and older), replicating the methods of Zhao et al 9 ; (2) the sex distribution of the study populations, and (3) studies of cohorts recruited before a median age of 51 years of age and followed up in health records until a median age of at least 60 years (ie, with stricter rules to further minimize lifetime selection biases). Because younger cohorts followed up to an age at which they may experience heart disease are less likely to be affected by lifetime selection biases, 9 we hypothesized that such studies would be less likely to show reduced mortality risks for low-volume drinkers. Finally, we reran the analyses using occasional drinkers (<1 drink per week) as the reference, for whom physiological health benefits are unlikely. Occasional drinkers are a more appropriate reference group, given evidence demonstrating that lifetime abstainers may be biased toward ill health. 10
The present study updates the systematic reviews and meta-analyses described above 8 by including studies published up to July 2021 to investigate whether the risk differed for subgroups. The study protocol was preregistered on the Open Science Framework. 11 Inclusion criteria, search strategy, study selection, data extraction, and statistical analytical methods of the study are summarized in later sections (see eAppendix in Supplement 1 for more details).
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline. 12 The review sought cohort studies of all-cause mortality and alcohol consumption. We identified all potentially relevant articles published up to July 31, 2021, regardless of language, by searching PubMed and Web of Science, through reference list cross-checking of previous meta-analyses (eFigure 1 in Supplement 1 ). There were 87 studies identified by Stockwell et al. 8 After inclusion of 20 new studies meeting inclusion criteria, there were a total of 107 cohort studies (eTable 1 in Supplement 1 ). 13 - 32
Three coders (J. Z., F. A., and J. C.) reviewed all eligible studies to extract and code data independently from all studies fulfilling the inclusion criteria. Data extracted included (1) outcome, all-cause mortality; (2) measures of alcohol consumption; (3) study characteristics, including cohort ages at recruitment and follow-up; (4) types of misclassification error of alcohol consumers and abstainers; (5) controlled variables in individual studies. Alcoholic drinks were converted into grams per day according to country-specific definitions if not otherwise defined. 33 , 34
We also assessed publication bias, heterogeneity, and confounding of covariates that might potentially affect the association of interest using several statistical approaches. 35 - 41 Relative risk (RR), including hazard ratios or rate ratios, were converted to natural log-transformed formats to deal with skewness. Publication bias was assessed through visual inspection of the funnel plot of log-RR of all-cause mortality due to alcohol consumption against the inverse standard error of log-RR 42 and Egger’s linear regression method. 36 We also plotted forest graphs of log-RR of all-cause mortality for any level of drinking to assess heterogeneity among studies. 42 The between-study heterogeneity of RRs were assessed using Cochran Q 37 and the I 2 statistic. 38 If heterogeneity was detected, mixed-effects models were used to obtain the summarized RR estimates. Mixed-effects regression analyses were performed in which drinking groups and control variables were treated as fixed-effects with a random study effect because of significant heterogeneity. 43
All analyses were weighted by the inverse of the estimated variance of the natural log relative risk. Variance was estimated from reported standard errors, confidence intervals, or number of deaths. The weights for each individual study were created using the inverse variance weight scheme and used in mixed regression analysis to get maximum precision for the main results of the meta-analysis. 42 In comparison with lifetime abstainers, the study estimated the mean RR of all-cause mortality for former drinkers (ie, now completely abstaining), current occasional (<9.1 g per week), low-volume (1.3-24.0 g per day), medium-volume (25.0-44.0 g per day), high-volume (45.0-64.0 g) and highest-volume drinkers (≥65.0 grams per day). The analyses adjusted for the potential confounding effects of study characteristics including the median age and sex distribution of study samples, drinker biases, country where a study was conducted, follow-up years and presence or absence of confounders. Analyses were also repeated using occasional drinkers as the reference group. We used t tests to calculate P values, and significance was set at .05. All statistical analyses were performed using SAS version 9.4 (SAS Institute) and the SAS MIXED procedure was used to model the log-transformed RR. 44 Data were analyzed from September 2021 to August 2022.
There were 724 estimates of the risk relationship between level of alcohol consumption and all-cause mortality from 107 unique studies 13 - 32 , 45 - 131 , including 4 838 825 participants and 425 564 deaths available for the analysis. Table 1 describes the sample characteristics of the metadata. Of 39 studies 13 , 15 , 18 , 21 , 23 - 26 , 29 , 31 , 45 - 47 , 49 , 50 , 52 - 54 , 57 - 59 , 62 , 64 , 70 , 80 , 81 , 85 , 87 , 91 , 94 , 96 , 100 , 104 , 107 , 118 , 124 , 125 , 127 , 130 reporting RR estimates for men and women separately, 33 14 , 17 , 48 , 51 , 61 , 63 , 66 , 68 , 69 , 72 , 76 , 79 , 83 , 84 , 86 , 88 , 90 , 92 , 93 , 97 , 98 , 101 , 103 , 105 , 109 - 111 , 113 - 115 , 119 , 120 , 128 were for males only, 8 16 , 65 , 73 , 99 , 102 , 108 , 112 , 123 for females only, and 30 13 , 19 - 22 , 26 - 30 , 32 , 55 , 56 , 67 , 71 , 74 , 75 , 77 , 78 , 82 , 84 , 89 , 95 , 106 , 116 , 117 , 121 , 122 , 126 , 129 for both sexes. Twenty-one studies 13 , 17 , 19 , 21 , 22 , 26 , 27 , 45 - 58 (220 risk estimates) were free from abstainer bias (ie, had a reference group of strictly defined lifetime abstainers). There were 50 studies 14 - 16 , 18 , 20 , 23 - 25 , 29 , 59 - 99 (265 risk estimates) with both former and occasional drinker bias; 28 studies 28 , 30 - 32 , 100 - 122 , 130 (177 risk estimates) with only former drinker bias; and 8 studies 123 - 129 , 131 (62 risk estimates) with only occasional drinker bias.
Unadjusted mean RR estimates for most study subgroups categorized by methods/sample characteristics showed markedly or significantly higher RRs for alcohol consumers as a group vs abstainers. Exceptions were for studies with less than 10 years of follow-up and those with some form of abstainer bias ( Table 1 ). Bivariable analyses showed that mortality risks for alcohol consumers varied considerably according to other study characteristics, such as quality of the alcohol consumption measure, whether unhealthy individuals were excluded at baseline, and whether socioeconomic status was controlled for ( Table 1 ).
No evidence of publication bias was detected either by inspection of symmetry in the funnel plot of log-RR estimates and their inverse standard errors (eFigure 2 in Supplement 1 ) or by Egger linear regression analysis (eTable 2 in Supplement 1 , all P > .05 for each study group). Significant heterogeneity was observed across studies for all drinking categories confirmed by both the Q statistic ( Q 723 = 5314.80; P < .001) and I 2 estimates (all >85.87%). (See eFigure 3 in Supplement 1 for forest plot of unadjusted risk estimates of mortality risks for the 20 newly identified studies).
Pooled unadjusted estimates (724 observations) showed significantly higher risk for former drinkers (RR, 1.22; 95% CI, 1.11-1.33; P = .001) and significantly lower risk for low-volume drinkers (RR, 0.85; 95% CI, 0.81-0.88; P = .001) compared with abstainers as defined in the included studies ( Table 2 ; eFigure 4 in Supplement 1 ). In the fully adjusted model, mortality RR estimates increased for all drinking categories, becoming nonsignificant for low-volume drinkers (RR, 0.93; 95% CI, 0.85-1.01; P = .07), occasional drinkers (>0 to <1.3 g of ethanol per day; RR, 0.96; 95% CI, 0.86-1.06; P = .41), and drinkers who drank 25 to 44 g per day (RR, 1.05; 95% CI, 0.96-1.14; P = .28). There was a significantly increased risk among drinkers who drank 45 to 64 g per day (RR, 1.19; 95% CI, 1.07-1.32; P < .001) and 65 or more grams (RR, 1.35; 95% CI, 1.23-1.47; P < .001). The Figure shows the changes in RR estimates for low-volume drinkers when removing each covariate from the fully adjusted model. In most cases, removing study-level covariates tended to yield lower risk estimates from alcohol use.
Table 2 presents the RR estimates when occasional drinkers were the reference group. In fully adjusted models, higher though nonsignificant mortality risks were observed for both abstainers and medium-volume drinkers (RR, 1.04; 95% CI, 0.94-1.16; P = .44 and RR, 1.09; 95% CI, 0.96-1.25; P = .19, respectively). There were significantly elevated risks for both high and higher volume drinkers (RR, 1.24; 95% CI, 1.07-1.44; P = .004 and RR, 1.41; 95% CI, 1.23-1.61; . P = 001, respectively).
As hypothesized, there was a significant interaction between cohort age and mortality risk ( P = .02; F 601 = 2.93) and so RR estimates for drinkers were estimated in analyses stratified by median age of the study populations at enrollment ( Table 3 ). In unadjusted and partially adjusted analyses, older cohorts displayed larger reductions in mortality risk associated with low-volume consumption than younger cohorts. However, in fully adjusted analyses with multiple covariates included for study characteristics, these differences disappeared. Younger cohorts also displayed greater mortality risks than older cohorts at higher consumption levels. Among studies in which participants were recruited at age 50 years or younger and followed up to age 60 years (ie, there was likely reduced risk of lifetime selection bias) higher RR estimates were observed for all drinking groups vs lifetime abstainers. These differences were significant in all drinking groups except low-volume drinkers (eTable 3 in Supplement 1 ).
Across all levels of alcohol consumption, female drinkers had a higher RR of all-cause mortality than males ( P for interaction = .001). As can be seen in Table 4 , all female drinkers had a significantly increased mortality risk compared with female lifetime nondrinkers (RR, 1.22; 95% CI, 1.02-1.46; P = .03). Compared with lifetime abstainers, there was significantly increased risk of all-cause mortality among male drinkers who drank 45 to 64 g per day (RR, 1.15; 95% CI, 1.03-1.28; P = .01) and drank 65 or more (RR, 1.34; 95% CI, 1.23-1.47; P < .001), and among female drinkers who drank 25 to 44 g per day (RR, 1.21; 95% CI, 1.08-1.36; P < .01), 45 to 64 g (RR, 1.34; 95% CI, 1.11-1.63; P < .01) and 65 or more grams (RR, 1.61; 95% CI, 1.44-1.80; P = .001).
In fully adjusted, prespecified models that accounted for effects of sampling, between-study variation, and potential confounding from former drinker bias and other study-level covariates, our meta-analysis of 107 studies found (1) no significant protective associations of occasional or low-volume drinking (moderate drinking) with all-cause mortality; and (2) an increased risk of all-cause mortality for drinkers who drank 25 g or more and a significantly increased risk when drinking 45 g or more per day.
Several meta-analytic strategies were used to explore the role of abstainer reference group biases caused by drinker misclassification errors and also the potential confounding effects of other study-level quality covariates in studies. 2 Drinker misclassification errors were common. Of 107 studies identified, 86 included former drinkers and/or occasional drinkers in the abstainer reference group, and only 21 were free of both these abstainer biases. The importance of controlling for former drinker bias/misclassification is highlighted once more in our results which are consistent with prior studies showing that former drinkers have significantly elevated mortality risks compared with lifetime abstainers.
In addition to presenting our fully adjusted models, a strength of the study was the examination of the differences in relative risks according to unadjusted and partially adjusted models, including the effect of removing individual covariates from the fully adjusted model. We found evidence that abstainer biases and other study characteristics changed the shape of the risk relationship between mortality and rising alcohol consumption, and that most study-level controls increased the observed risks from alcohol, or attenuated protective associations at low levels of consumption such that they were no longer significant. The reduced RR estimates for occasional or moderate drinkers observed without adjustment may be due to the misclassification of former and occasional drinkers into the reference group, a possibility which is more likely to have occurred in studies of older cohorts which use current abstainers as the reference group. This study also demonstrates the degree to which observed associations between consumption and mortality are highly dependent on the modeling strategy used and the degree to which efforts are made to minimize confounding and other threats to validity.
It also examined risk estimates when using occasional drinkers rather than lifetime abstainers as the reference group. The occasional drinker reference group avoids the issue of former drinker misclassification that can affect the abstainer reference group, and may reduce confounding to the extent that occasional drinkers are more like low-volume drinkers than are lifetime abstainers. 2 , 8 , 132 In the unadjusted and partially adjusted analyses, using occasional drinkers as the reference group resulted in nonsignificant protective associations and lower point estimates for low-volume drinkers compared with significant protective associations and higher point estimates when using lifetime nondrinkers as the reference group. In the fully adjusted models, there were nonsignificant protective associations for low-volume drinkers whether using lifetime abstainers or occasional drinkers as the reference group, though this was only a RR of 0.97 for the latter.
Across all studies, there were few differences in risk for studies when stratified by median age of enrollment above or below age 56 years in the fully adjusted analyses. However, in the subset of studies who enrolled participants aged 50 years or younger who were followed for at least 10 years, occasional drinkers and medium-volume drinkers had significantly increased risk of mortality and substantially higher risk estimates for high- and higher-volume consumption compared with results from all studies. This is consistent with our previous meta-analysis for CHD, 9 in which younger cohorts followed up to older age did not show a significantly beneficial association of low-volume consumption, while older cohorts, with more opportunity for lifetime selection bias, showed marked, significant protective associations.
Our study also found sex differences in the risk of all-cause mortality. A larger risk of all-cause mortality for women than men was observed when drinking 25 or more grams per day, including a significant increase in risk for medium-level consumption for women that was not observed for men. However, mortality risk for mean consumption up to 25 g per day were very similar for both sexes.
A number of limitations need to be acknowledged. A major limitation involves imperfect measurement of alcohol consumption in most included studies, and the fact that consumption in many studies was assessed at only 1 point in time. Self-reported alcohol consumption is underreported in most epidemiological studies 133 , 134 and even the classification of drinkers as lifetime abstainers can be unreliable, with several studies in developed countries finding that the majority of self-reported lifetime abstainers are in fact former drinkers. 135 , 136 If this is the case, the risks of various levels of alcohol consumption relative to presumed lifetime abstainers are underestimates. Merely removing former drinkers from analyses may bias studies in favor of drinkers, since former drinkers may be unhealthy, and should rightly be reallocated to drinking groups according to their history. However, this has only been explored in very few studies. Our study found that mortality risk differed significantly by cohort age and sex. It might be that the risk is also higher for other subgroups, such as people living with HIV, 137 a possibility future research should investigate.
The number of available studies in some stratified analyses was small, so there may be limited power to control for potential study level confounders. However, the required number of estimates per variable for linear regression can be much smaller than in logistic regression, and a minimum of at least 2 estimates per variable is recommended for linear regression analysis, 138 suggesting the sample sizes were adequate in all models presented. It has been demonstrated that a pattern of binge (ie, heavy episodic) drinking removes the appearance of reduced health risks even when mean daily volume is low. 139 Too few studies adequately controlled for this variable to investigate its association with different outcomes across studies. Additionally, our findings only apply to the net effect of alcohol at different doses on all-cause mortality, and different risk associations likely apply for specific disease categories. The biases identified here likely apply to estimates of risk for alcohol and all diseases. It is likely that correcting for these biases will raise risk estimates for many types of outcome compared with most existing estimates.
This updated meta-analysis did not find significantly reduced risk of all-cause mortality associated with low-volume alcohol consumption after adjusting for potential confounding effects of influential study characteristics. Future longitudinal studies in this field should attempt to minimize lifetime selection biases by not including former and occasional drinkers in the reference group, and by using younger cohorts (ie, age distributions that are more representative of drinkers in the general population) at baseline.
Accepted for Publication: February 17, 2023.
Published: March 31, 2023. doi:10.1001/jamanetworkopen.2023.6185
Correction: This article was corrected on May 9, 2023, to fix errors in the Figure and Supplement.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Zhao J et al. JAMA Network Open .
Corresponding Author: Jinhui Zhao, PhD, Canadian Institute for Substance Use Research, University of Victoria, PO Box 1700 STN CSC, Victoria, BC V8Y 2E4, Canada ( [email protected] ).
Author Contributions: Drs Zhao and Stockwell had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zhao, Stockwell, Naimi, Churchill, Sherk.
Acquisition, analysis, or interpretation of data: Zhao, Stockwell, Naimi, Clay.
Drafting of the manuscript: Zhao, Stockwell, Clay.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Zhao, Churchill.
Obtained funding: Zhao, Stockwell, Sherk.
Administrative, technical, or material support: Zhao, Stockwell, Naimi.
Supervision: Zhao, Stockwell, Naimi.
Conflict of Interest Disclosures: Dr Stockwell reported receiving personal fees from Ontario Public Servants Employees Union for expert witness testimony and personal fees from Alko outside the submitted work. Dr Sherk reported receiving grants from Canadian Centre on Substance Use and Addiction (CCSA) during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was partly funded by the CCSA as a subcontract for a Health Canada grant to develop guidance for Canadians on alcohol and health.
Role of the Funder/Sponsor: Health Canada had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. CCSA staff conducted a preliminary search to identify potentially relevant articles but did not participate in decisions about inclusion/exclusion of studies, coding, analysis, interpretation of results or approving the final manuscript.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We gratefully acknowledge contributions by Christine Levesque, PhD (CCSA), and Nitika Sanger, PhD (CCSA), who conducted a preliminary literature search for potentially relevant articles. We also acknowledge the leadership of Drs Catherine Paradis, PhD (CCSA), and Peter Butt, MD (University of Saskatchewan), who cochaired the process of developing Canada’s new guidance on alcohol and health, a larger project which contributed some funds for the work undertaken for this study. We are grateful to Fariha Alam, MPH (Canadian Institute for Substance Use and Research), for her help coding the studies used in this study. None of them received any compensation beyond their normal salaries for this work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
The effects of these drinks on the human body are not fully understood, which is why research into their negative effects has increased. 4.1. Effects on the Cardiovascular System. Effects on the cardiovascular system appear to be the most studied of all the side effects of these substances, due to their potentially fatal properties.
In the United States, overweight and obesity are chronic diseases that contribute to excess morbidity and mortality. Despite public health efforts, these disorders are on the rise, and their consequences are burgeoning. 1 The Centers for Disease Control and Prevention report that during 2017 to 2018, the prevalence of obesity in the United States was 42.4%, which was increased from the ...
Health risks of warming of 1.5° C, 2° C, ... Advancing Australia's role in climate change and health research. ... Environmental Health: A Position Paper From the American College of Physicians.
Almost 35 years ago, the Office of the Surgeon General of the United States Health Service reviewed over 7000 research papers on the topic of smoking and health, and publicly recognized the role of smoking in various diseases, including lung cancer. ... Cardiovascular health risks of smoke-exposed women are of particular concern. Although the ...
As a leading behavioral risk factor for numerous health outcomes, smoking is a major ongoing public health challenge. Although evidence on the health effects of smoking has been widely reported ...
Möller L, Schuetzle D, Autrup H. Future research needs associated with the assessment of potential human health risks from exposure to toxic ambient air pollutants. Environ Health Perspect. (1994) 102(Suppl. 4):193-210. 10.1289/ehp.94102s4193 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Background Sedentary lifestyle is a major risk factor for noncommunicable diseases such as cardiovascular diseases, cancer and diabetes. It has been estimated that approximately 3.2 million deaths each year are attributable to insufficient levels of physical activity. We evaluated the available evidence from Cochrane systematic reviews (CSRs) on the effectiveness of exercise/physical activity ...
Objective To better understand the state of research on the effects of climate change on human health, including exposures, health conditions, populations, areas of the world studied, funding sources, and publication characteristics, with a focus on topics that are relevant for populations at risk.Design Cross sectional study.Data sources The National Institute of Environmental Health Sciences ...
This discussion paper concludes with recommendations for priority actions to address the mental health consequences of climate change. ... Tunstall S, Tapsell S, Green C, Floyd P, George C. The health effects of flooding: social research results from England and Wales. J Water Health. 2006;4(3):365-80.
In models adjusting for potential confounding effects of sampling variation, former drinker bias, and other prespecified study-level quality criteria, the meta-analysis of all 107 included studies found no significantly reduced risk of all-cause mortality among occasional (>0 to <1.3 g of ethanol per day; relative risk [RR], 0.96; 95% CI, 0.86 ...