An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Muhammad F. Hashmi ; Mary E. Cataletto .
Affiliations
Last Update: May 3, 2024 .
- Continuing Education Activity
Asthma is a chronic inflammatory respiratory condition characterized by hallmark symptoms of intermittent dyspnea, cough, and wheezing. However, due to the nonspecific nature of these symptoms, distinguishing asthma from other respiratory illnesses can sometimes be challenging. A confirmed diagnosis of asthma relies on consistent respiratory symptoms and the identification of variable expiratory airflow obstruction documented on spirometry. Clinicians prioritize symptom control and prevention of future exacerbations through tailored treatment, considering symptom frequency, severity, and potential risks in a step-wise approach. Early recognition and intervention of asthma exacerbations are crucial to prevent the progression of asthma to severe, life-threatening stages. Fatalities related to asthma highlight missed opportunities in recognizing disease severity and escalating therapy, emphasizing the critical role of continual patient education and routine symptom control assessment for successful long-term management.
The development of asthma, often presenting in childhood, involves a complex interplay of genetic and environmental factors associated with atopy. Researchers strive to develop predictive systems for identifying individuals at risk of continued symptoms into adulthood. Despite significant advancements in understanding the underlying genetic loci, environmental triggers, and risk factors, clinical strategies remain lacking to mitigate the risks of persistent asthma development into adolescence and adulthood. This activity covers the epidemiology, pathophysiology, and assessment of asthma, along with initiating pharmacological treatment and developing monitoring strategies tailored for adolescents and adults. These strategies closely align with evidence-based recommendations from the National Asthma Education and Prevention Program and the Global Initiative for Asthma.
- Identify the hallmark symptoms of asthma, including dyspnea, cough, and wheezing.
- Implement evidence-based treatment strategies for asthma management, considering individual patient characteristics and preferences.
- Assess asthma severity, control, and exacerbation risk regularly during follow-up visits.
- Collaborate with interdisciplinary healthcare team members to optimize asthma care and patient outcomes.
- Introduction
Asthma is a prevalent chronic inflammatory respiratory condition affecting millions of people worldwide and presents substantial challenges in both diagnosis and management. This respiratory condition is characterized by inflammation of the airways, causing intermittent airflow obstruction and bronchial hyperresponsiveness. The hallmark asthma symptoms include coughing, wheezing, and shortness of breath, which can be frequently exacerbated by triggers ranging from allergens to viral infections. The prevalence and severity of asthma are determined by a complex interplay between genetic and environmental factors. Despite treatment advancements, disparities persist in asthma care, with variations in access to diagnosis, treatment, and patient education across different demographics.
The development of asthma, often presenting in childhood, is associated with other atopic features, such as eczema and hay fever. [1] [2] [3] Severity varies from intermittent symptoms to life-threatening airway closure. Healthcare professionals establish a definitive diagnosis through patient history, physical examination, pulmonary function testing, and appropriate laboratory testing. Spirometry with a post-bronchodilator response (BDR) is the primary diagnostic test. Treatment focuses on providing continued education, routine symptom assessment, access to fast-acting bronchodilators, and appropriate controller medications tailored to disease severity.
Asthma manifests with diverse phenotypes, likely influenced by intricate interactions between genetic and environmental factors. [4] [5] Genomewide association studies have linked childhood-onset asthma to markers near the ORMDL sphingolipid biosynthesis regulator 3 ( ORMDL3 ) and gasdermin B ( GSDMB ) genes on chromosome 17q21, encoding ORM1-like protein 3 and gasdermin-like protein. [6] Other associations include genes such as interleukin-33 ( IL33 ), IL-1 receptor-like 1 ( IL1R1 ) genes, and a novel susceptibility locus at the IF-inducible protein X ( PYHIN1 ) gene, particularly affecting individuals of African descent. [7]
The EVE Consortium also identifies a susceptibility locus for thymic stromal lymphopoietin ( TSLP ), an epithelial cell–derived cytokine implicated in asthma-related inflammation initiation. [8] Asthma patients exhibit higher TSLP expression in their airways compared to healthy controls. Additional genetic loci involved in asthma include major histocompatibility complex class II DQ α1 ( HLA-DQA1 ), HLA-DQB1 antisense RNA 1 ( HLA-DQB1 ), Toll-like receptor 1 ( TLR1 ), IL-6 receptor ( IL6R ), zona pellucida-binding protein 2 ( ZPBP2 ), and gasdermin A ( GSDMA ).
Genetics may also be pivotal in asthma treatment. The hydroxy-δ-5-steroid dehydrogenase, 3-beta- and steroid δ-isomerase 1 ( HSD3B1 ) genotype is associated with glucocorticoid resistance among patients. In addition, single-nucleotide polymorphisms in protein kinase cGMP-dependent 1 ( PRKG1 ) and SPATA13 antisense RNA 1 ( SPATA13-AS1 ) are associated with BDR in Black children. [9]
Differing concordance rates among monozygotic twins suggest that exposure to environmental factors has an essential role in the development of asthma. Specific alleles have different effects depending on the environmental exposures. For example, exposure to secondhand smoke associates variations in the N -acetyltransferase 1 ( NAT1 ) gene with the development of asthma in children. A study involving 983 children with single-nucleotide polymorphisms related to ORMDL3 and GSDMB at chromosome locus 17q21 reveals that the same genotype poses genetic risk while also offering environmental protection. [10]
Risk Factors
Risk factors for asthma development encompass exposures throughout a patient's lifespan, including the perinatal period. The most substantial known risk factor is atopy, which is characterized by the genetic tendency to produce specific immunoglobulin E (IgE) antibodies in response to common environmental allergens. Nearly one-third of children with atopy will develop asthma later in life.
Prenatal and Perinatal Factors
Prematurity is the most crucial risk factor influencing asthma incidence during this period. [11] [12] [13] [14] Preterm birth, occurring before 36 weeks, is associated with an elevated risk of asthma throughout childhood, adolescence, and adulthood. Researchers posit that impaired lung development in preterm infants, even in those without early respiratory complications, increases the long-term risk of asthma. [15] Exposure to maternal smoking during pregnancy causes diminished pulmonary function in newborns and an increased probability of developing childhood asthma. Moreover, smoking during pregnancy correlates with several adverse pregnancy outcomes, including premature delivery, further elevating the asthma risk.
The incidence of childhood asthma increases with a maternal age of 20 or younger and decreases with a maternal age of 30 or older. Maternal diet during pregnancy holds significance, with researchers suggesting that vitamin D deficiency contributes to early-life wheezing and asthma primarily by impacting the immune function of various cell types, notably dendritic and T regulatory cells. Additionally, vitamin D plays a role in fetal lung development. [16] [17] Although some studies present conflicting findings regarding the association between maternal vitamin D levels and childhood asthma, a meta-analysis of 2 large studies indicates that maternal vitamin D intake offers protection against wheezing or asthma in offspring up to the age of 3. [16]
The Copenhagen Prospective Studies on Asthma in Childhood (COPSAC2010) reveals that 17% of children born to mothers with diets high in omega-3 polyunsaturated fatty acids developed persistent wheeze or asthma during the first 3 years of life compared to nearly 24% in the group with diets high in omega-6 polyunsaturated fatty acids. Vitamins E and C and zinc may also have protective effects. Administering vitamin C at a dose of 500 mg/d to pregnant mothers appears to offer protection against the harmful effects of tobacco exposure. Offspring of mothers who receive vitamin C supplementation exhibit a wheezing incidence of 28%, while those without vitamin C supplementation have a higher incidence of 47%. [18] [19]
Wheezing caused by viral infections, particularly respiratory syncytial virus and human rhinovirus, may predispose infants and young children to develop asthma later in life. In addition, early-life exposure to air pollution, including combustion by-products from gas-fired appliances and indoor fires, obesity, and early puberty, also increases the risk of asthma.
The most significant risk factors for adult-onset asthma include tobacco smoke, occupational exposure, and adults with rhinitis or atopy. Studies also suggest a modest increase in asthma incidence among postmenopausal women taking hormone replacement therapy.
Furthermore, the following factors can contribute to asthma and airway hyperreactivity:
- Exposure to environmental allergens such as house dust mites, animal allergens (especially from cats and dogs), cockroach allergens, and fungi
- Physical activity or exercise
- Conditions such as hyperventilation, gastroesophageal reflux disease, and chronic sinusitis
- Hypersensitivity to aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), as well as sulfite sensitivity
- Use of β-adrenergic receptor blockers, including ophthalmic preparations
- Exposure to irritants such as household sprays and paint fumes
- Contact with various high- and low-molecular-weight compounds found in insects, plants, latex, gums, diisocyanates, anhydrides, wood dust, and solder fluxes, which are associated with occupational asthma
- Emotional factors or stress
Aspirin-Exacerbated Respiratory Disease
Aspirin-exacerbated respiratory disease (AERD) is a condition characterized by a combination of asthma, chronic rhinosinusitis with nasal polyposis, and NSAID intolerance. Patients with AERD present with upper and lower respiratory tract symptoms after ingesting aspirin or NSAIDs that inhibit cyclooxygenase-1 (COX-1). This condition arises from dysregulated arachidonic acid metabolism and the overproduction of leukotrienes involving the 5-lipoxygenase and cyclooxygenase pathways. AERD affects approximately 7% of adults with asthma.
Occupational-Induced Asthma
Two types of occupational asthma exist based on their appearance after a latency period:
- Occupational asthma triggered by workplace sensitizers results from an allergic or immunological process associated with a latency period induced by both low- and high-molecular-weight agents. High-molecular-weight substances, such as flour, contain proteins and polysaccharides of plant or animal origin. Low-molecular-weight substances, like formaldehyde, form a sensitizing neoantigen when combined with a human protein.
- Occupational asthma caused by irritants involves a nonallergic or nonimmunological process induced by gases, fumes, smoke, and aerosols.
- Epidemiology
The worldwide incidence of asthma is estimated to affect 260 million individuals. [20] Recent studies examining asthma prevalence across 17 countries reveal varying rates, ranging from 3.4% to 6% for adults and children in India, Taiwan, Kosovo, Nigeria, and Russia, and higher rates of 17% to 33% for Honduras, Costa Rica, Brazil, and New Zealand. [21] Despite data showing the death rate consistently declining for asthma between 2001 and 2015, asthma continues to account for approximately 420,000 deaths per year. [22] Factors such as under-prescription of inhaled glucocorticoids and limited access to emergency medical care or specialist care all play a role in asthma-related deaths.
Asthma prevalence in the United States differs among demographic groups, including age, gender, race, and socioeconomic status. The United States Centers for Disease Control and Prevention (CDC) estimates that around 25 million Americans are currently affected by asthma. Among individuals younger than 18, boys exhibit a higher prevalence compared to girls, while among adults, women are more commonly affected than men. Additionally, asthma prevalence is notably higher among Black individuals, with a prevalence of 10.1%, compared to White individuals at 8.1%. Hispanic Americans generally have a lower prevalence of 6.4%, except for those from Puerto Rico, where the prevalence rises to 12.8%. Moreover, underrepresented minorities and individuals living below the poverty line experience the highest incidence of asthma, along with heightened rates of asthma-related morbidity and mortality.
Similar to worldwide data, the mortality rate of asthma in the United States has also undergone a consistent decline. The current mortality rate is 9.86 per million compared to 15.09 per million in 2001. However, mortality rates remain consistently higher for Black patients compared to their White counterparts. According to the CDC, from 1999 to 2016, asthma death rates among adults aged 55 to 64 were 16.32 per 1 million persons, 9.95 per 1 million for females, 9.39 per 1 million for individuals who were not Hispanic or Latino, and notably higher at 25.60 per 1 million for Black patients.
- Pathophysiology
Asthma is a syndrome characterized by diverse underlying mechanisms and involves intricate interactions among inflammatory and resident airway cells. These mechanisms lead to airway inflammation, intermittent airflow obstruction, and bronchial hyperresponsiveness (see Image. Pathophysiology of Asthma).
Airway Inflammation
The activation of mast cells by cytokines and other mediators plays a pivotal role in the development of clinical asthma. Following initial allergen inhalation, affected patients produce specific IgE antibodies due to an overexpression of the T-helper 2 subset (Th2) of lymphocytes relative to the Th1 type. Cytokines produced by Th2 lymphocytes include IL-4, IL-5, and IL-13, which promote IgE and eosinophilic responses in atopy. Once produced, these specific IgE antibodies bind to receptors on mast cells and basophils. Upon additional allergen inhalation, allergen-specific IgE antibodies on the mast cell surface undergo cross-linking, leading to rapid degranulation and the release of histamine, prostaglandin D2 (PGD2), and cysteinyl leukotrienes C4 (LTC4), D4 (LTD4), and E4 (LTE4). [23] [24] This triggers contraction of the airway smooth muscle within minutes and may stimulate reflex neural pathways. Subsequently, an influx of inflammatory cells, including monocytes, dendritic cells, neutrophils, T lymphocytes, eosinophils, and basophils, may lead to delayed bronchoconstriction several hours later.
Airflow Obstruction
The narrowing of the airway lumen throughout the tracheobronchial tree is caused by the contraction of airway smooth muscle, thickening of the airway wall due to edema, mucus plugging in the airways, and airway remodeling, which collectively contributes to varying levels of airflow obstruction.
Mediators such as histamine and leukotrienes, released from inflammatory cells or through reflex neural pathways, trigger the contraction and relaxation of airway smooth muscle. The precise mechanism leading to airway hyperresponsiveness, characterized by an excessive tightening of the airway's smooth muscles in response to various physical, chemical, or environmental triggers, remains unclear. Some researchers propose alterations in breathing patterns where smooth muscles contract excessively or fail to relax adequately during deep breaths as a potential explanation.
Airway remodeling, which involves thickening of the basement membrane, deposition of collagen, and shedding of epithelial cells, can lead to irreversible changes in the airways. This process accelerates the decline in lung function, particularly in individuals with severe and early-onset asthma. [25] In addition, remodeling contributes to the heightened bronchial sensitivity observed in asthma.
Arachidonic acid metabolism by the enzyme 5-lipoxygenase (5-LO) leads to the generation of leukotrienes, which serve as potent bronchoconstrictors. The metabolism of arachidonic acid by the 2 cyclooxygenase (COX) isoforms—COX-1 and COX-2—generates prostaglandins and thromboxanes. PGD2 is a potent bronchodilator, while PGE2 suppresses the production of leukotrienes. Patients with AERD have dysregulated arachidonic acid metabolism, causing decreased production of PGE2 and loss of control of leukotriene production. [26]
Patients with occupational-induced asthma can undergo an immunologically mediated response similar to those without occupational-induced asthma. Alternatively, others may present with nonimmunological occupational asthma. The possible underlying mechanisms of the nonimmunological form are denudation of the airway epithelium, direct β-2 adrenergic receptor inhibition, or elaboration of substance P by injured sensory nerves.
- History and Physical
The 4 cardinal symptoms associated with asthma are wheezing, cough (often worse at night), shortness of breath, and chest tightness. Individuals may experience 1 or more of these symptoms. Asthma symptoms typically occur intermittently, lasting for hours to days, and resolve upon the removal of triggers or the administration of asthma medications. Nighttime exacerbation of symptoms or onset triggered by exercise, cold air, or allergen exposure suggests asthma. In contrast to exertional dyspnea, which manifests shortly after beginning exertion and resolves within 5 minutes of cessation, exercise-induced asthma symptoms typically emerge around 15 minutes into activity and dissipate within 30 to 60 minutes afterward. Patients may also have a history of other forms of atopy, such as eczema and hay fever.
During patient history-taking, healthcare professionals should inquire about particular triggers that exacerbate symptoms. Common household triggers include dust, animals, and infestations of rodents and cockroaches. Some individuals may experience intermittent asthma symptoms related to their work shifts. A strong family history of asthma and allergies, or a personal history of atopic conditions and childhood asthma symptoms, suggests asthma in patients exhibiting suggestive symptoms.
Physical Examination
During physical examination, widespread, high-pitched wheezes are a characteristic finding associated with asthma. However, wheezing is not specific to asthma and is typically absent between acute exacerbations. Findings suggestive of a severe asthma exacerbation include tachypnea, tachycardia, a prolonged expiratory phase, reduced air movement, difficulty speaking in complete sentences or phrases, discomfort when lying supine due to breathlessness, and adopting a "tripod position." [27] The use of the accessory muscles of breathing during inspiration and pulsus paradoxus are additional indicators of a severe asthma attack.
Healthcare professionals may identify extrapulmonary findings that support the diagnosis of asthma, such as pale, boggy nasal mucous membranes, posterior pharyngeal cobblestoning, nasal polyps, and atopic dermatitis. Nasal polyps should prompt further inquiry about anosmia, chronic sinusitis, and aspirin sensitivity to evaluate for AERD. Although AERD is uncommon in children or adolescents, the presence of nasal polyps in a child with lower respiratory disease should prompt an evaluation for cystic fibrosis. Clubbing, characterized by bulbous fusiform enlargement of the distal portion of a digit, is not associated with asthma and should prompt evaluation for alternative diagnoses. Please see StatPearls' companion resource, " Nail Clubbing ," for further information.
Intermittent symptoms consistent with asthma, in addition to wheezing observed during physical examination, strongly indicate asthma. Confirming the diagnosis involves the exclusion of alternative diagnoses and a demonstration of variable airflow limitation, usually seen in spirometry.
Spirometry assesses forced expiratory volume in 1 second (FEV 1 ) and forced vital capacity (FVC) by measuring a maximal inhalation followed by rapid and forceful exhalation into a spirometer. Asthma typically presents as an obstructive pattern on spirometry, indicated by a reduced FEV 1 to FVC ratio. [28] Additionally, a visual examination of the expiratory flow-volume loop can reveal an obstructive pattern. A scooped, concave appearance in the expiratory portion of the flow-volume loop indicates diffuse intrathoracic airflow obstruction characterizes asthma. In rare cases where complete exhalation is impossible, the FEV 1 /FVC ratio may appear normal, falsely suggesting a restrictive pattern if not assessed along with flow-time curves.
Patients showing airflow limitations on spirometry receive 2 to 4 puffs of a short-acting bronchodilator like albuterol, followed by repeat spirometry in 10 to 15 minutes. According to the European Respiratory Society/American Thoracic Society guidelines, a positive BDR is determined by a change in FEV 1 or FVC compared to their predicted value. Clinicians calculate the patient's BDR using the formula:
BDR=([Post-bronchodilator value – Pre-bronchodilator value] × 100) / Predicted value of either FEV 1 or FVC
Increases exceeding 10% are considered significant. [28]
According to the Global Initiative for Asthma, a significant BDR is indicated by an increase in the FEV 1 of 12% or 200 mL or more. In addition, the slow vital capacity, or the maximal amount of air exhaled in a relaxed expiration from full inspiration to residual volume over 15 seconds, may also be helpful when the FVC is reduced and airway obstruction is present. During slow exhalation, airway narrowing is less pronounced, and the patient can produce a larger vital capacity. In cases of restrictive disease, both slow and fast exhalations result in reduced vital capacity.
Spirometry results may be normal in asymptomatic individuals or those with cough-variant asthma. Bronchodilator responsiveness is evident in asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, non-cystic fibrosis bronchiectasis, and bronchiolitis. However, patients with asthma may yield false negative results if they are on chronic controller medications, exhibit underlying airway remodeling, have minimal symptoms during testing, or have recently used bronchodilators before the test. Ideally, clinicians should conduct baseline spirometry before commencing treatment. [29] [30]
Bronchoprovocation Testing
During bronchoprovocation testing, clinicians induce bronchoconstriction using inhaled methacholine or mannitol, exercise, or eucapnic hyperventilation of dry air. This testing method can be beneficial for patients presenting with atypical symptoms or an isolated cough. Patients receive incremental doses of the provocative agent followed by spirometry to generate a dose-response curve. A fall in FEV 1 of 20% or more from baseline with the standard dose of methacholine or a decline of 15% or more with the standard dose of hypertonic saline, mannitol, or hyperventilation indicates a positive test. [31] Clinicians may also conduct additional provocative testing using exercise, aspirin, and exposure to environmental triggers encountered in the workplace.
Peak Flow Meter
Although consistent reductions of 20% during symptomatic periods, followed by a gradual return to baseline as symptoms resolve, indicate asthma, clinicians typically use peak flow measurement to monitor patients with known asthma rather than for initial diagnosis. To measure peak flow, the patient takes a maximal breath and seals the peak flow meter between their lips before blowing forcefully for 1 to 2 seconds. Please see StatPearls' companion resource, " Peak Flow Measurement ," for additional information regarding peak flow measurement and its clinical significance in the evaluation and management of asthma.
Patients repeat this process 3 times, recording the highest reading as the current peak flow measurement. Patients can compare their recorded values to established graphs based on age and height for adults and height for adolescents to determine their predicted value. Notably, reduced peak flow values are not specific to asthma. Patients with either an obstructive or restrictive pattern on spirometry can have decreased peak flow values. Additionally, the accuracy of results is highly contingent on patient effort.
Exhaled Nitric Oxide
Eosinophilic airway inflammation causes an upregulation of nitric oxide synthase in the respiratory mucosa, leading to elevated nitric oxide levels in exhaled breath. In certain asthma patients, the fractional exhaled nitric oxide (FE NO ) surpasses levels observed in individuals without asthma. A FE NO of measurement exceeding 40 to 50 ppb can aid in confirming an asthma diagnosis.
Pulse Oximetry
Pulse oximetry can help assess the severity of an asthma attack or monitor for deterioration. Notably, pulse oximetry measurements may exhibit a lag, and the physiological reserve of many patients implies that a declining oxygen level on pulse oximetry is a late stage, indicating an increasingly unwell or peri-arrest patient.
No specific laboratory tests are necessary for diagnosing asthma. However, patients who present with a severe asthma exacerbation should undergo a complete blood count to evaluate eosinophil levels and check for anemia, which may be the underlying cause of the patient's dyspnea. A significantly elevated eosinophil count should prompt further investigation for conditions, including parasitic infections such as Strongyloides , drug reactions, and syndromes characterized by pulmonary infiltrates with eosinophilia. These syndromes include allergic bronchopulmonary aspergillosis, eosinophilic granulomatosis with polyangiitis, and hypereosinophilic syndrome (see Image. Allergic Bronchopulmonary Aspergillosis on CT Scan).
Non-smoking patients who present with irreversible airflow obstruction should undergo serum α1-antitrypsin level testing to rule out emphysema caused by homozygous α1-antitrypsin deficiency. Allergy testing may prove beneficial for patients experiencing symptoms upon exposure to specific allergens. Clinicians should obtain total serum IgE levels in patients with moderate-to-severe persistent asthma, particularly when considering treatment with anti-IgE monoclonal antibodies or when there is suspicion of allergic bronchopulmonary aspergillosis. Please refer to the Treatment/Management section for further details on anti-IgE monoclonal antibodies.
Chest radiographs in asthma patients are often normal; however, during acute exacerbations, abnormal findings such as hyperinflation, pneumomediastinum, and bronchial thickening may be observed (see Image. A Chest Radiograph Depicting Asthma). A chest radiograph is recommended for patients aged 40 or older with new-onset, moderate-to-severe asthma to rule out conditions that can mimic asthma, such as a mediastinal mass with tracheal compression or heart failure.
Additional indications for chest radiography include patients experiencing symptoms that are difficult to control, fever, chronic purulent sputum production, persistently localized wheezing, hemoptysis, weight loss, clubbing, inspiratory crackles, significant hypoxemia, and moderate or severe airflow obstruction that does not reverse with bronchodilators. High-resolution computed tomography is necessary to clarify any abnormalities noted on chest radiographs or for patients with other suspected conditions that may not be well visualized on routine radiographs.
Evaluation During an Acute Exacerbation
Each patient should undergo a rapid assessment of their vital signs, including oxygen saturation. Measuring the peak flow can indicate the severity of the exacerbation and monitor the response to therapy. Predicted peak flow measurements vary based on age and height; however, a peak flow below 200 L/min indicates severe obstruction except in patients aged 65 or older or with very short stature. A peak flow measurement below 50% predicted or the patient's personal best is considered severe, while between 50% and 70% is considered moderate. Chest radiographs are not uniformly necessary unless the diagnosis of acute asthma exacerbation is uncertain, the patient requires hospitalization, or evidence of a comorbid condition is present.
Identification of Patients at Risk of Fatal or Near-Fatal Asthma
Most asthma-related deaths are preventable if risk factors are identified and addressed early. Major risk factors that place patients at high risk for future fatal asthma exacerbations include:
- A recent history of poorly controlled asthma
- A prior history of near-fatal asthma
- A history of endotracheal intubation for asthma
- A history of intensive care unit admission for asthma
Minor risk factors include exposure to aeroallergens and tobacco smoke, illicit drug use, older patients, aspirin sensitivity, long duration of asthma, and frequent hospitalizations for asthma-related issues.
- Treatment / Management
Patient Education
Multiple sources of patient education are available. According to the National Asthma Education and Prevention Program's Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, personalized education from the patient's primary clinician is especially impactful. Studies reveal that such education reduces the number of asthma exacerbations and hospitalizations. Healthcare professionals should provide culturally specific asthma education that includes understanding asthma and its symptoms, identifying the patient's specific triggers, and strategies for their avoidance. Each patient should understand how to properly use an inhaler and be familiar with medications that serve as rescue options, those used for symptom control, and those that may fulfill both roles. Clinicians should inquire about any obstacles hindering medication adherence and work collaboratively with patients to overcome concerns or barriers, thus enhancing overall adherence.
Although the data on effectiveness are limited, a general consensus among experts exists that individuals with asthma should possess a personalized "action plan" to follow at home (please refer to the link to an action plan download in the Deterrence and Patient Education section). This action plan provides a structured maintenance medication regimen and delineates steps to take when symptoms exacerbate. Clinicians develop an action plan based on symptoms or peak flow readings and divide it into 3 zones—green, yellow, and red.
Patients in the green zone are asymptomatic, with peak flows at 80% or higher than their personal best. They feel well and continue with their long-term control medication. Peak flow readings falling within the yellow zone range between 50% and 79% of the patient's personal best, accompanied by symptoms such as coughing, wheezing, and shortness of breath, which begin to interfere with activity levels. In the red zone, patients experience peak flow readings below 50% of their best, severe shortness of breath, and an inability to perform everyday activities.
Asthma Severity
Guidelines established by the National Asthma Education and Prevention Program (NAEPP) and the Global Initiative for Asthma (GINA) determine therapy based on the frequency and severity of asthma symptoms, the degree of respiratory impairment, and the risk of future exacerbations. Risk factors contributing to future exacerbations include frequent asthma symptoms, a history of intensive care unit admission for asthma, obesity, poor medication adherence, chronic rhinosinusitis, and a low FEV 1 . The severity categories and treatment guidelines vary based on age. This activity will address asthma severity and management in adolescents and adults aged 12 or older. Please see StatPearls' companion resource, " Pediatric Asthma ," for additional information regarding the treatment of asthma in infants and children.
Every patient should have access to a bronchodilator with a rapid onset of action. Traditionally, this has been a short-acting β-agonist (SABA) such as albuterol. However, GINA recommends a low-dose glucocorticoid/formoterol inhaler, such as 80 to 160 mcg budesonide/4.5 mcg formoterol inhaled by mouth 1 or 2 times daily, for asthma symptoms. Notably, this is an off-label indication for this preparation.
Treatment progresses in a stepwise manner, with the highest severity category in which the patient experiences any symptoms, designating the treatment category from which the patient receives treatment (see Image. Asthma Severity Classification by National Asthma Education and Prevention Program). Tables 1 and 2 below include the NAEPP and GINA asthma severity classifications and treatment initiation guidelines based on the patient's symptoms and lung function.
Table 1. National Asthma Education and Prevention Program: Expert Panel Working Group Initial Asthma Therapy in Adolescents and Adults.
Abbreviations: FEV 1 , forced expiratory volume in 1 second; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; SABA, short-acting β-agonist.
Table 2. Global Initiative for Asthma Initial Asthma Therapy in Adolescents and Adults.
Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β-agonist.
Routine follow-up every 1 to 6 months is necessary to ensure adequate symptom management. Upon reevaluation, patients facing inadequate asthma symptom management, exacerbations necessitating systemic glucocorticoids, or those at high risk of exacerbation on their current therapy level should escalate to the next level of therapy. Therapy adjustments proceed incrementally until symptoms are adequately managed. After maintaining control for 3 to 6 months, clinicians may consider gradual therapy reduction following the stepwise protocols outlined by GINA or NAEPP guidelines.
Severe Asthma
Adults and adolescents with severe asthma that remains uncontrolled despite Step 4 recommended therapy should receive a LAMA, such as tiotropium, alongside their inhaled glucocorticoid and LABA regimen. Clinicians should direct these patients for phenotypic assessment and consideration for biological therapy options. Anti-IgE monoclonal antibody therapy with omalizumab may be helpful for those still experiencing inadequate control and possessing documented sensitivity to a perennial allergy with IgE levels ranging between 30 and 700 IU/mL.
Patients with severe eosinophilic asthma who are not adequately controlled can utilize mepolizumab and reslizumab, monoclonal antibodies against IL-5, benralizumab, a monoclonal antibody against the IL-5 receptor α-subunit, and dupilumab a monoclonal antibody against the IL-4 receptor α-subunit. Tezepelumab is a human monoclonal IgG2-λ antibody that binds to TSLP, preventing its interaction with the TSLP receptor complex. [32]
Acute Exacerbation
Patients experiencing an acute asthma exacerbation may manage symptoms at home or need urgent medical care depending on their symptom severity and risk factors for fatal asthma. These risk factors include prior life-threatening exacerbations, exacerbations despite glucocorticoid use, more than 1 asthma-related hospitalization or 3 emergency room visits in the past year, and comorbidities such as cardiovascular or chronic lung disease. Immediate medical attention is warranted for patients showing significant breathlessness, inability to speak beyond short phrases, reliance on accessory muscles, or peak flow measurements at 50% or less of their baseline measurement.
All patients require a fast-acting β-agonist. Potential options include the LABA formoterol combined with ICS, the SABA albuterol combined with budesonide, or albuterol alone. Combination with ICS is the preferred choice. Albuterol dosing is 2 to 4 puffs from a metered dose inhaler (MDI) at home and 4 to 8 puffs in the office with a valved holding chamber or spacer every 20 minutes for 1 hour as needed. Albuterol may also be nebulized. ICS-formoterol dosing is 1 to 2 puffs every 20 minutes for 1 hour as required, with a maximum of 8 puffs per day.
Patients whose symptoms improve after administering a bronchodilator and whose peak flow returns to 80% of their baseline or better can continue to manage their symptoms at home. Oral glucocorticoids equivalent to 40 to 60 mg prednisone daily for 5 to 7 days are warranted for the following patients:
- Those experiencing recurrent symptoms over the following 1 to 2 days.
- Those whose peak flow remains less than 80% of their normal baseline (high-dose ICSs are an alternative).
- If they do not improve after 1 to 3 doses of a fast-acting bronchodilator.
- If they have recently completed a course in OCS.
- Those who are on a maximal dose of controller medications.
Patients with a peak flow value of 50% or lower despite administering a bronchodilator or continuing to worsen should seek immediate medical care while continuing to administer their fast-acting bronchodilator.
Office management is similar to home management, with the addition that according to GINA guidelines, all patients with oxygen saturation below 90% should receive oxygen to maintain saturation above 92% or 95% for pregnant individuals. Albuterol treatment can be administered via an MDI or nebulizer, with a dosage of 4 to 8 puffs or 2.5 to 5 mg every 20 minutes for 1 hour, respectively. Research comparing the efficacy of an MDI combined with a valved-holding chamber to nebulizer delivery, both administering the same β-agonist but with significantly lower doses via MDI, demonstrates similar enhancements in lung function and risk reduction for hospitalization. [33] [34] [35]
If oral glucocorticoids are unavailable, intramuscular steroids such as triamcinolone suspension (40 mg/mL) 60 to 100 mg can be an alternative. However, it is noteworthy that intramuscular glucocorticoids have a delayed onset of action of 12 to 36 hours. Patients meeting certain criteria such as a respiratory rate of 30 breaths per minute, a heart rate of more than 120 bpm, a continued peak flow of less than 50% predicted, oxygen saturation of less than 90%, or the inability to speak in full sentences should be transferred to the emergency department.
Patients who can be sent home from the office should have their controller medications advanced in 1 step. In addition, it is essential to review the correct use of their inhaler, discuss trigger avoidance strategies, ensure they have an asthma action plan, and emphasize the importance of adhering to their controller medication.
Emergency Department Care
Within the first hour, patients should receive 3 treatments of an inhaled SABA, such as albuterol, via a nebulizer or MDI, followed by repeat dosing every 1 to 4 hours. In addition to a SABA, patients with severe asthma exacerbations should also receive inhaled ipratropium, a short-acting muscarinic antagonist (SAMA), at a dosage of 500 µg by nebulization or 4 to 8 puffs by MDI, every 20 minutes for 3 doses, and then hourly as needed for up to 3 hours. Current guidelines recommend discontinuing SAMA therapy once the patient requires hospital admission, except in specific cases such as refractory asthma requiring treatment in the intensive care unit, concurrent treatment with monoamine oxidase inhibitors due to potential increased toxicity from sympathomimetic therapy due to impaired drug metabolism, presence of COPD with an asthmatic component, or asthma triggered by β-blocker therapy.
As with outpatient management, patients also receive glucocorticoids equivalent to 40 to 60 mg of prednisone daily for 5 to 7 days. A systematic review reveals no difference between a higher dose and a longer course when compared to a lower dose with a shorter course of prednisone or prednisolone. [36] Oral and intravenous glucocorticoids have equivalent effects when administered in comparable doses. Intravenous steroids are necessary for patients with impending or actual respiratory arrest or who are intolerant of oral glucocorticoids. Some clinicians administer higher doses of glucocorticoids for severe asthma exacerbations based on their expert opinion and concern that a lower dose might be insufficient in a critically ill patient.
Magnesium sulfate
Per GINA guidelines, magnesium is not recommended for routine use in asthma exacerbations. However, a 1-time dose of 2 g given intravenously over 20 minutes reduces hospitalization rates in adults with an FEV 1 less than 25% to 30% predicted on presentation and in those who fail to respond to initial treatment and continue to have hypoxemia. Nebulized MgSO 4 is not beneficial in the management of an acute asthma exacerbation.
A Cochrane Database review in 2014 concluded that a single infusion of intravenous MgSO 4 for patients not responding to conventional therapy results in improved lung functions and fewer hospital admissions. [37] However, in a recent systematic review, the comparison of the same studies, eliminating those involving children and those containing only abstracts, revealed conflicting results. The review examined the effects of intravenous and nebulized MgSO 4 . Although 3 out of 9 studies addressing treatment with intravenous MgSO 4 found a significant effect on lung function compared to placebo, these results are not statistically significant. [38] Only 2 of the 8 studies investigating hospital admission rates reveal a significant effect of MgSO 4 . [38] Conversely, 6 of the 9 studies investigating treatment with nebulized MgSO 4 compared to placebo reveal a favorable effect on the FEV 1 and peak expiratory flow rate. [38] These results somewhat contradict the Cochrane Database review conducted in 2014, which evaluated the same studies. [37]
An additional study reveals a small benefit of adding inhaled magnesium to inhaled albuterol plus ipratropium in reducing hospital admissions but no significant improvement in peak expiratory flow when added to inhaled albuterol plus ipratropium or inhaled albuterol alone. [39]
Intubation or Noninvasive Ventilation
Indications for intubation and mechanical ventilation or noninvasive ventilation include the following:
- Slowing of the respiratory rate
- Depressed mental status
- Inability to maintain respiratory effort
- Inability to cooperate with the administration of inhaled medications
- Worsening hypercapnia and associated respiratory acidosis
- Inability to maintain oxygen saturation above 92% despite face mask supplemental oxygen
A 1- to 2-hour trial of bilevel noninvasive positive pressure ventilation is appropriate for patients with an acute asthma exacerbation, but clinicians should maintain a low threshold for intubation. [40] [41]
Additional Therapies
Occasionally, nonstandard therapies, such as ketamine, halothane, helium-oxygen mixtures, extracorporeal membrane oxygenation, and parenteral terbutaline, can be helpful for certain patients. However, these therapies are not routinely utilized due to limited evidence of efficacy. The indication for parenteral epinephrine is asthma associated with anaphylaxis and angioedema.
All patients who are smokers should be educated on the benefits of smoking cessation and provided with appropriate support and resources. Empiric antibiotics are not recommended since most infections triggering asthma exacerbations are viral. According to both GINA and NAEPP guidelines, intravenous methylxanthines such as theophylline are deemed ineffective and are no longer part of the standard of care. [42]
- Differential Diagnosis
The differential diagnoses for asthma include the following conditions:
- Bronchiectasis
- Bronchiolitis
- Chronic obstructive pulmonary disease
- Chronic sinusitis
- Cystic fibrosis
- α1-antitrypsin deficiency
- Aspergillosis
- Exercise-induced anaphylaxis
- Foreign body aspiration
- Heart failure
- Gastroesophageal reflux disease
- Tracheomalacia
- Pulmonary embolism
- Pulmonary eosinophilia
- Sarcoidosis
- Upper respiratory tract infection
- Vocal cord dysfunction
- Eosinophilic granulomatosis with polyangiitis
- Bronchogenic carcinoma
- Post-viral tussive syndrome
- Eosinophilic bronchitis
- Cough induced by angiotensin-converting enzyme inhibitors
- Bordetella pertussis infection
- Interstitial lung disease
- Recurrent oropharyngeal aspiration
The development and prognosis of asthma involve a complex interplay of genetic and environmental factors. Social determinants of health, such as poor housing quality and indoor and outdoor pollution, profoundly impact asthma prognosis. In the United States, asthma is a chronic illness characterized by a significant racial and ethnic disparity in both prevalence and prognosis. Underrepresented racial and ethnic minorities, as well as individuals living below the poverty line, experience higher morbidity rates, increased emergency room visits, hospitalizations, and mortality due to asthma. [43] [44] Additionally, lack of access to healthcare—whether due to difficulties in accessing clinicians or lack of insurance—further exacerbates prognosis-related challenges.
The international asthma mortality rate reaches as high as 0.86 deaths per 100,000 persons in certain countries. The overall prognosis is predominantly linked to lung function, with mortality rates 8 times higher among individuals in the bottom 25% of lung function. Several factors contribute to a poorer prognosis, including inadequate asthma management, age 40 or older, a history of more than 20 pack-years of cigarette smoking, blood eosinophilia, and FEV1 of 40% to 69% of predicted values
- Complications
The complications related to asthma include disease-related complications and adverse effects of glucocorticoids, LTRA, and endotracheal intubation. The following list contains complications associated with asthma:
- Decline in lung function
- Osteoporosis
- Adrenal suppression
- Hypertension
- Peptic ulcer
- Sleep disorders
- Obstructive sleep apnea
- Mood disorders
- Cardiac arrest
- Respiratory failure or arrest
- Pneumothorax
- Aspiration [45]
- Consultations
Healthcare professionals should seek consultation with an asthma specialist in pulmonology or allergy when the diagnosis of asthma is uncertain, the patient's symptoms remain poorly controlled, medication adverse effects become intolerable, or the patient experiences frequent exacerbations. Accessing appropriate specialist care aids in excluding alternate diagnoses, determining the need for additional diagnostic testing, and effectively escalating medical therapy.
- Deterrence and Patient Education
Patient education plays a pivotal role in the effective management of asthma by clinicians. To deter exacerbations and improve patient outcomes, clinicians should emphasize the importance of adherence to medication regimens, avoidance of triggers, and regular monitoring of symptoms. Educating patients about asthma triggers, such as allergens, air pollution, and tobacco smoke, can empower them to make informed lifestyle choices. Furthermore, clinicians should highlight the significance of having an asthma action plan, which outlines steps to take during worsening symptoms or exacerbations. See the National Heart and Lung Institute's website, " Asthma Action Plan ," for a printable version of an action plan.
Patient education should also prioritize the recognition of early warning signs of an asthma attack and prompt seeking of medical attention when necessary. Routine follow-up visits for patients with active asthma are recommended, occurring every one to six months, contingent on the severity of asthma and adequacy of control. During these follow-up visits, clinicians should assess asthma control, lung function, exacerbations, inhaler technique, adherence, adverse effects of medication, quality of life, and patient satisfaction with care. By instilling a comprehensive understanding of asthma management strategies and fostering proactive patient involvement, clinicians can significantly reduce the burden of asthma and enhance patient well-being.
- Enhancing Healthcare Team Outcomes
Asthma is characterized by complex pathophysiology involving airway inflammation, intermittent airflow obstruction, and bronchial hyperresponsiveness. The condition presents various signs and symptoms, such as wheezing, coughing, shortness of breath, and chest tightness. Wheezing may not always be present, particularly in cases primarily affecting small airways, and its absence does not exclude asthma. Additionally, a cough might be the sole symptom, especially one that occurs or worsens at night. Diagnostic evaluation involves spirometry, assessing lung function parameters such as FEV1 and FVC, measuring peak flow, and possibly conducting bronchoprovocation testing in some individuals.
Treatment strategies include trigger avoidance, ensuring access to rescue medications, and personalized pharmacological interventions, with inhaled corticosteroids being the preferred controller medication. Patient education, regular assessment of symptom control, and adherence to treatment plans are crucial components in effectively managing asthma. Adequate patient readiness and preparation, including the development of an asthma action plan, help minimize illness severity and optimize patient outcomes by promoting self-management and reducing healthcare utilization.
Enhancing patient-centered care, outcomes, patient safety, and team performance in asthma management demands a strategic approach. Each healthcare team member should possess the necessary clinical expertise to diagnose and treat asthma effectively, which involves interpreting spirometry findings and customizing treatment plans according to individual patient needs. Adhering to evidence-based guidelines and protocols will ensure uniform practices across healthcare settings.
Effective interprofessional communication enables the exchange of information, collaborative decision-making, and seamless care transitions. Each healthcare team member, including physicians, advanced care practitioners, nurses, pharmacists, respiratory therapists, and social workers, contributes unique skills to asthma care, further enriching interdisciplinary collaboration. By fostering a culture of collaboration, communication, and coordination, healthcare professionals can deliver comprehensive, patient-centered asthma care, decreasing morbidity and mortality and enhancing team performance.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Pathophysiology of Asthma. Figure A displays the location of the lungs and airways in the body. Figure B shows a cross section of a normal airway. Figure C illustrates a cross section of an airway during asthma symptoms National Institutes of Health
A Chest Radiograph Depicting Asthma. The image depicts both anterior and lateral radiographs of a patient with asthma. The image highlights the presence of pneumomediastinum and increased bronchovascular markings. Contributed by H Shulman, MD
Allergic Bronchopulmonary Aspergillosis on CT Scan. Computed tomography (CT) images reveal bronchiectasis in both upper lobes of a patient with bronchial asthma, indicative of allergic bronchopulmonary aspergillosis. Contributed by M Salahuddin, MD
Asthma Severity Classification by The National Asthma Education and Prevention Program. Contributed by R Chabra, DO
Disclosure: Muhammad Hashmi declares no relevant financial relationships with ineligible companies.
Disclosure: Mary Cataletto declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Hashmi MF, Cataletto ME. Asthma. [Updated 2024 May 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- [Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)]. [Zhonghua Jie He He Hu Xi Za Zh...] [Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)]. Pulmonary Function and Clinical Respiratory Physiology Committee of Chinese Association of Chest Physicians, Chinese Thoracic Society, Pulmonary Function Group of Respiratory Branch of Chinese Geriatric Society. Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12; 47(2):101-119.
- The effectiveness of school-based family asthma educational programs on the quality of life and number of asthma exacerbations of children aged five to 18 years diagnosed with asthma: a systematic review protocol. [JBI Database System Rev Implem...] The effectiveness of school-based family asthma educational programs on the quality of life and number of asthma exacerbations of children aged five to 18 years diagnosed with asthma: a systematic review protocol. Walter H, Sadeque-Iqbal F, Ulysse R, Castillo D, Fitzpatrick A, Singleton J. JBI Database System Rev Implement Rep. 2015 Oct; 13(10):69-81.
- Pediatric Asthma. [StatPearls. 2024] Pediatric Asthma. Lizzo JM, Goldin J, Cortes S. StatPearls. 2024 Jan
- Review Diagnosis and Treatment of Asthma in Nonpregnant Women. [J Midwifery Womens Health. 2019] Review Diagnosis and Treatment of Asthma in Nonpregnant Women. Esden J, Pesta-Walsh N. J Midwifery Womens Health. 2019 Jan; 64(1):18-27. Epub 2018 Nov 28.
- Review Low-grade disease activity in early life precedes childhood asthma and allergy. [Dan Med J. 2016] Review Low-grade disease activity in early life precedes childhood asthma and allergy. Chawes BL. Dan Med J. 2016 Aug; 63(8).
Recent Activity
- Asthma - StatPearls Asthma - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Diagnosis and Management of Asthma in Adults: A Review
Affiliations.
- 1 Division of Allergy and Clinical Immunology, University of Texas Medical Branch, Galveston.
- 2 Department of Preventive Medicine and Community Health, University of Texas Medical Branch, Galveston.
- 3 Division of Pulmonary Critical Care and Sleep, Department of Internal Medicine, University of Texas Medical Branch, Galveston.
- PMID: 28719697
- DOI: 10.1001/jama.2017.8372
Importance: Asthma affects about 7.5% of the adult population. Evidence-based diagnosis, monitoring, and treatment can improve functioning and quality of life in adult patients with asthma.
Observations: Asthma is a heterogeneous clinical syndrome primarily affecting the lower respiratory tract, characterized by episodic or persistent symptoms of wheezing, dyspnea, and cough. The diagnosis of asthma requires these symptoms and demonstration of reversible airway obstruction using spirometry. Identifying clinically important allergen sensitivities is useful. Inhaled short-acting β2-agonists provide rapid relief of acute symptoms, but maintenance with daily inhaled corticosteroids is the standard of care for persistent asthma. Combination therapy, including inhaled corticosteroids and long-acting β2-agonists, is effective in patients for whom inhaled corticosteroids alone are insufficient. The use of inhaled long-acting β2-agonists alone is not appropriate. Other controller approaches include long-acting muscarinic antagonists (eg, tiotropium), and biological agents directed against proteins involved in the pathogenesis of asthma (eg, omalizumab, mepolizumab, reslizumab).
Conclusions and relevance: Asthma is characterized by variable airway obstruction, airway hyperresponsiveness, and airway inflammation. Management of persistent asthma requires avoidance of aggravating environmental factors, use of short-acting β2-agonists for rapid relief of symptoms, and daily use of inhaled corticosteroids. Other controller medications, such as long-acting bronchodilators and biologics, may be required in moderate and severe asthma. Patients with severe asthma generally benefit from consultation with an asthma specialist for consideration of additional treatment, including injectable biologic agents.
Publication types
- Administration, Inhalation
- Adrenal Cortex Hormones / adverse effects
- Adrenal Cortex Hormones / therapeutic use
- Adrenergic beta-Agonists / adverse effects
- Adrenergic beta-Agonists / therapeutic use
- Airway Obstruction / physiopathology
- Anti-Asthmatic Agents / adverse effects
- Anti-Asthmatic Agents / therapeutic use*
- Asthma / diagnosis*
- Asthma / drug therapy*
- Asthma / physiopathology
- Biological Products / therapeutic use
- Bronchial Hyperreactivity / physiopathology
- Drug Therapy, Combination
- Inflammation
- Muscarinic Antagonists / therapeutic use
- Adrenal Cortex Hormones
- Adrenergic beta-Agonists
- Anti-Asthmatic Agents
- Biological Products
- Muscarinic Antagonists
Grants and funding
- UL1 TR000071/TR/NCATS NIH HHS/United States
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Asthma: epidemiology, etiology and risk factors
Padmaja subbarao , md msc, piush j mandhane , md phd, malcolm r sears , mb chb.
- Author information
- Copyright and License information
Correspondence to: Prof. Malcolm R. Sears, Firestone Institute for Respiratory Health, St. Joseph’s Healthcare, 50 Charlton Ave. E., Hamilton ON L8N 4A6; fax 905 521-6132; [email protected]
Asthma is one of the most common chronic conditions affecting both children and adults, yet much remains to be learned of its etiology. This paper evolved from the extensive literature review undertaken as part of a proposal for a longitudinal birth cohort study to examine risk factors for the development of allergy and asthma in early childhood.
Although genetic predisposition is clearly evident, gene-by-environment interaction probably explains much of the international variation in prevalence rates for allergy and asthma. Environmental factors such as infections and exposure to endotoxins may be protective or may act as risk factors, depending in part on the timing of exposure in infancy and childhood. Some prenatal risk factors, including maternal smoking, have been firmly established, but diet and nutrition, stress, use of antibiotics and mode of delivery may also affect the early development of allergy and asthma. Later in childhood, putative risk factors include exposure to allergens, breastfeeding (which may initially protect and then increase the risk of sensitization), family size and structure, and sex and gender. In adulthood, recurrence of childhood asthma may be just as common as new-onset asthma, which may have an occupational basis. A better understanding of these risk factors may eventually lead to opportunities for primary prevention of asthma.
This paper arose from an extensive literature review undertaken in developing the Canadian Healthy Infant Longitudinal Development (CHILD) study, a multicentre national observational study that is currently in progress. The study, which will eventually recruit 5000 pregnant women, has the aim of determining the environmental, host, genetic and psychosocial risk factors for development of allergy and asthma in children. Although not a systematic review, the examination of epidemiologic risk factors in the development of asthma presented here began in 2004 with a search of MEDLINE, using the Medical Subject Heading (MeSH) terms “asthma,” “longitudinal” and “cohort study.” One of us (P.S.) reviewed the abstracts of all studies identified in the search, excluding those without at least one objective outcome measure and those in which the primary outcome measure was not asthma. Studies examining the same outcome measure were tabulated but not combined, since most did not consider exactly the same outcome at the same age. We then performed specific searches to fill gaps in the information gathered via the original search, specifically nutrition, sex and gender effects, and novel environmental exposures. The review was updated in July 2008.
Although the present article includes some references to adult asthma, its primary focus is the epidemiology of and risk factors for this condition in children. A more extensive summary of the literature review for the Canadian Healthy Infant Longitudinal Development study has been published elsewhere. 1
Epidemiology of asthma: an overview
The recent substantial increase in the reported prevalence of asthma worldwide ( Figure 1 ) has led to numerous studies of the prevalence and characteristics of this condition. 2 Foremost among these are 2 major international initiatives that have collected data using validated questionnaires, one among children, the International Study of Asthma and Allergies in Childhood, 3 and the other among young adults, the European Community Respiratory Health Survey. 4 Follow-up investigations for both of these studies 5 , 6 have examined temporal trends within and across populations. During a mean of 7 years following phase I of the International Study of Asthma and Allergies in Childhood, which in most participating countries was conducted between 1991 and 1993, the prevalence of asthma was stable or decreased in some areas of the world but increased substantially in many other areas, especially among children 13–14 years of age ( Figure 2 ). 5
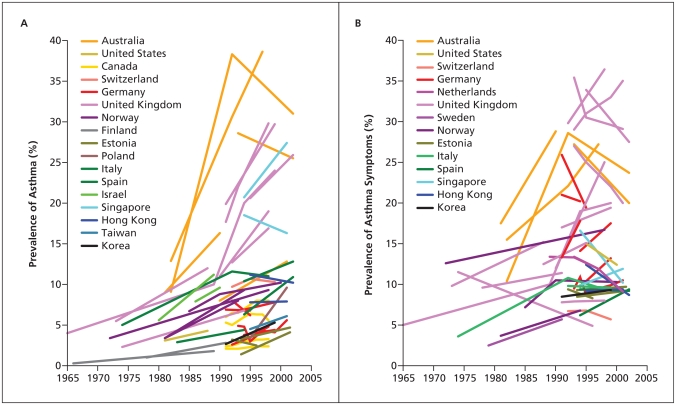
Changes in prevalence of diagnosed asthma (A) and asthma symptoms (B) over time among children and young adults. Reproduced, with permission, from Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006;355:2226–35. Copyright 2006 Massachusetts Medical Society. 2
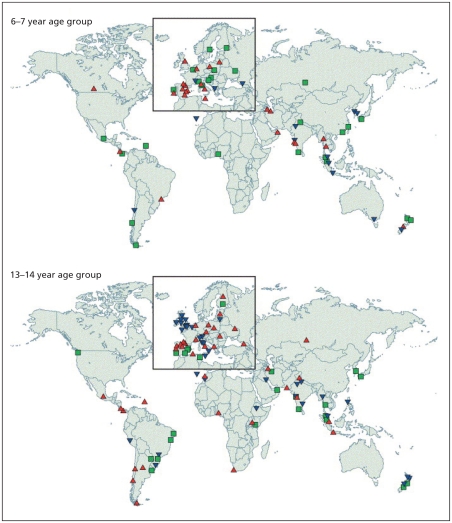
Annual changes in worldwide prevalence of asthma symptoms among children 6–7 years old and 13–14 years old, over a mean of 7 years following phase I of the International Study of Asthma and Allergies in Childhood (which in most participating countries was conducted between 1991 and 1993). Blue triangles identify locations where prevalence was reduced by at least 1 standard error (SE) per year, green squares identify locations where there was little change in prevalence (i.e., change of less than 1 SE per year) and red triangles identify locations where prevalence increased by at least 1 SE per year. Reproduced from The Lancet, Vol. 368, Asher MI, Montefort S, Bjorksten B, et al.; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Pages 733–43, copyright 2006, with permission from Elsevier. 5
Cross-sectional population-based studies such as these are highly dependent on recognition of symptoms, so they do not necessarily reflect the true heterogeneity of asthma. However, a wide variation in prevalence rates has been documented: studies of both children and adults have revealed low prevalence rates (2%–4%) in Asian countries (especially China and India) and high rates (15%–20%) in the United Kingdom, Canada, Australia, New Zealand and other developed countries. 3 – 6
Observations of migrating populations 7 and of Germany after reunification 8 have strongly supported the role of local environmental factors, including allergens but likely many lifestyle factors as well, in determining the degree of expression of asthma within genetically similar populations. A recent analysis of data from the International Study of Asthma and Allergies in Childhood, comparing data from Vancouver, Canada, with data from centres in China, showed significant differences in prevalence rates between children of similar genetic ancestry living in different environments, with evidence for an effect of duration of residence in the new environment. 9 Prevalence rates for asthma among children 13–14 years old were lowest for Chinese children born and studied in China, intermediate for Chinese children who had migrated during their lifetime to Canada and highest for Chinese children who had been born in Canada. In addition, the prevalence rate for the third of these groups was still lower than among non-Chinese children in the same environment. Together, these results strongly suggested gene-by-environment interactions.
Local and national studies have also provided insights into the epidemiology of exacerbations of asthma. For example, epidemics of asthma exacerbations in Barcelona, Spain, were eventually linked to exposure to atmospheric soybean dust released during cargo handling at the local port. 10 The highly predictable annual epidemic of asthma exacerbations in school-age children in the northern hemisphere every September, peaking some 17 days after the return to school, appears to be predominantly driven by seasonal rhinovirus infection, probably compounded by other risk factors for asthma exacerbations, including reduction in use of asthma controller therapy over the summer months, exposure to seasonal allergens and possibly the stress of returning to school. 11 , 12
Complementing these cross-sectional studies are longitudinal epidemiologic studies in a variety of populations and countries, which have allowed examination of risk factors predicting the development, persistence, remission or relapse of childhood asthma. One such population-based birth cohort study in Dunedin, New Zealand, which had a high retention rate, examined outcomes of childhood asthma at age 26 years. 13 Female sex, airway hyperresponsiveness in mid and later childhood, and sensitization to house dust mites were all significantly and independently related to the likelihood of persistence of childhood asthma to early adulthood. Early age of onset of wheezing symptoms was predictive of relapse after remission, as were airway hyperresponsiveness and allergy to house dust mites. That study and others have clearly demonstrated the tracking of characteristics of asthma from childhood to adulthood, including severity and impairment of lung function.
Etiology of and risk factors for asthma
Asthma comprises a range of heterogeneous phenotypes that differ in presentation, etiology and pathophysiology. The risk factors for each recognized phenotype of asthma include genetic, environmental and host factors. Although a family history of asthma is common, it is neither sufficient nor necessary for the development of asthma. 14
The substantial increases in the incidence of asthma over the past few decades and the geographic variation in both base prevalence rates and the magnitude of the increases support the thesis that environmental changes play a large role in the current asthma epidemic. Furthermore, environmental triggers may affect asthma differently at different times of a person’s life, and the relevant risk factors may change over time.
Short-term studies of risk factors may suggest a lower likelihood of asthma, whereas the same factors may be associated with greater risk if follow-up is more prolonged. This pattern may relate to overlap between different wheezing phenotypes in early childhood, only some of which persist as asthma in later childhood and adulthood. Because of this phenomenon, we examine here the risk factors for persistent asthma at different ages, specifically the prenatal period, infancy, childhood and, briefly, adulthood.
Family and twin studies have indicated that genetics plays an important role in the development of asthma and allergy, 15 likely through several genes of moderate effect (i.e., genes associated with relative risks in the range of 1.2–2). 16 , 17
Genome-wide linkage studies and case–control studies have identified 18 genomic regions and more than 100 genes associated with allergy and asthma in 11 different populations. In particular, there are consistently replicated regions on the long arms of chromosomes 2, 5, 6, 12 and 13. Association studies of unrelated individuals have also identified more than 100 genes associated with allergy and asthma, 79 of which have been replicated in at least one further study. 18 A recent genome-wide association study 19 identified a new gene, ORMDL3 , that exhibited a highly significantly association with asthma ( p < 10 −12 ) (for single nucleotide polymorphism rs8067378, odds ratio 1.84, 95% confidence interval 1.43–2.42) a finding that has now been replicated in several populations. 20 , 21
Extensive heterogeneity in the genetic basis of asthma, and in gene-by-environment interactions, is likely. Failure to identify and precisely quantify environmental exposures and their timing may account for some of the difficulty that researchers have had in replicating genetic associations.
Prenatal risk factors
Risk factors in the prenatal period are multifactorial. Assessment is complicated by the variety of wheezing conditions that may occur in infancy and childhood, only some of which evolve to classical asthma.
Prenatal tobacco smoke
Prenatal maternal smoking has been consistently associated with early childhood wheezing, 22 – 25 and there is a dose–response relation between exposure and decreased airway calibre in early life. 26 , 27 Prenatal maternal smoking is also associated with increased risks of food allergy, 24 cytokine responses in the cord blood 28 , 29 and concentrations of nitric oxide in exhaled air in newborns. 30 Studies have shown a clear prenatal effect of smoking; this effect is increased when combined with postnatal smoke exposure.
Diet and nutrition
Observational studies examining prenatal nutrient levels or dietary interventions and the subsequent development of atopic disease have focused on foods with anti-inflammatory properties (e.g., omega-3 fatty acids) and antioxidants such as vitamin E and zinc. Several studies have demonstrated that higher intake of fish or fish oil during pregnancy is associated with lower risk of atopic disease (specifically eczema and atopic wheeze) up to age 6 years. 31 – 33 Similarly, higher prenatal vitamin E and zinc levels have been associated with lower risk of development of wheeze up to age 5 years. 34 – 36 However, no protective effect against the development of atopic disease in infants has been shown for maternal diets that excluded certain foods (e.g., cow’s milk, eggs) during pregnancy. 37 – 40 The authors of 2 recent studies 41 , 42 reported an inverse relation of maternal vitamin D levels with wheeze in early life, but no relation with atopy or symptoms in later life.
A number of animal models have suggested that prenatal maternal stress acts through regulation of the offspring’s hypothalamic–pituitary–adrenal axis to decrease cortisol levels, which may affect the development of an allergic phenotype. Although there is a correlation between caregiver stress early in the infant’s life and higher levels of immunoglobulin E in the infant 43 – 45 and early wheezing, 46 no studies to date have shown an association with asthma. 47 , 48
Antibiotic use
The association between prenatal antibiotic treatment and subsequent development of atopic disease has been examined in 2 ways: with treatment as a dichotomous predictor (i.e., any antibiotic use) and by number of courses of antibiotics during pregnancy. Longitudinal cohort studies examining any antibiotic use showed a greater risk of persistent wheeze and asthma in early childhood 49 , 50 and a dose–response relation between number of antibiotic courses and risk of wheeze or asthma. 49 , 51
Mode of delivery
Development of atopy was 2 to 3 times more likely among infants delivered by emergency cesarean section, 29 , 52 – 56 although no such association occurred with elective cesarean section. 29 , 52 , 53 , 56 – 59 Potential reasons for these findings include maternal stress and differences in the infant’s gut microflora associated with different modes of delivery.
Risk factors in childhood
Phenotypes of asthma.
Although some 50% of preschool children have wheezing, only 10%–15% have a diagnosis of “true” asthma by the time they reach school age. 13 , 60 Commonly described phenotypes in early infancy and childhood are transient wheezing, nonatopic wheezing, late-onset wheezing and persistent wheezing. 61 Only transient wheezing in early infancy has been well characterized, with decreased airflow rates on pulmonary function testing at birth, 56 , 60 , 62 onset of wheezing within the first year and resolution by mid-childhood with no lasting effects on pulmonary function.
The other 3 phenotypes have been described primarily by age of onset in cohort studies, and their genesis in early infancy is largely unknown. The majority of children with persistent wheezing (in whom asthma will subsequently be diagnosed) experience their first symptoms before age 3. By 3 years, they have abnormal lung function that persists to adulthood, 13 , 60 , 61 and by adolescence, most have atopy. Of children with nonatopic and late-onset wheezing, some experience remission, whereas others experience persistent symptoms and atopy. 63
Distinguishing among these different phenotypes in early childhood is critical to understanding the role of risk factors and their timing in early infancy.
Breastfeeding
The influence of breastfeeding on the risk of childhood atopy and asthma remains controversial. The following represents observational data accumulated to date. Some studies have shown protection, 64 – 66 whereas others have reported higher rates of allergy and asthma among breastfed children. 67 , 68 A meta-analysis 69 and several individual studies 66 , 70 showed that exclusive breastfeeding for at least 3 months was associated with lower rates of asthma between 2 and 5 years of age, with the greatest effect occurring among those with a parental history of atopy. One of the difficulties in interpreting these data lies in differentiating viral-associated wheeze in childhood from development of atopic asthma. In a longitudinal birth cohort study, breastfeeding was associated with a higher risk of atopic asthma in later childhood, with the greatest in fluence occurring among those with a maternal history of atopy. 67 , 68 , 71
The influence of avoiding nutritional allergens during breastfeeding is also controversial. In some studies, exclusion of milk, eggs and fish from the maternal diet was associated with decreased atopic dermatitis in infancy, 72 , 73 but other studies found no association. 40 , 74 , 75 Studies following children to 4 years of age have demonstrated no effect of maternal dietary restriction during lactation on the subsequent development of atopic diseases, including asthma. 76
Lung function
Decreased airway calibre in infancy has been reported as a risk factor for transient wheezing, 60 perhaps related to prenatal and postnatal exposure to environmental tobacco smoke. 26 , 27 Furthermore, the presence of airways with decreased calibre has been associated with increased bronchial responsiveness and increased symptoms of wheeze. 26 Several studies have suggested an association between reduced airway function in the first few weeks of life and asthma in later life. 62 , 77 The magnitude of the effect of this risk factor in isolation (i.e., without concomitant allergy) is unclear; perhaps individuals with smaller airways require less stimulus (i.e., airway inflammation) before symptoms become apparent.
Children with wheezing (and diagnosed asthma) persisting to adulthood have a fixed decrement in lung function as early as age 7 or 9 years. 13 , 78 Recent studies of preschool children have documented abnormal lung function in children with persistent wheezing as young as age 3 years. 61 However, some infants in whom persistent wheezing develops have normal lung function shortly after birth, which suggests a critical period of exposures within the first few years of life, before the development of these persistent abnormalities in expiratory flows. 60 , 79 In contrast, infants who exhibit early transient wheezing have decreased airflow shortly after birth. 60 , 80 Maternal smoking with in utero nicotine exposure has been correlated with this type of lung dysfunction, 26 , 27 , 60 but the effects of other exposures have been less well studied.
Family structure
Family size and the number and order of siblings may affect the risk of development of asthma. The hygiene hypothesis posits that exposure of an infant to a substantial number of infections and many types of bacteria stimulates the developing immune system toward nonasthmatic phenotypes. 81 , 82 This may be exemplified in the real world by large family size, whereby later-born children in large families would be expected to be at lower risk of asthma than first-born children, because of exposure to their older siblings’ infections.
Although this theory has been supported by some studies of allergy prevalence, 83 , 84 it has been partially refuted by recent studies of asthma prevalence suggesting that although large family size (more than 4 children) is associated with a decreased risk of asthma, birth order is not involved. 85 , 86 Furthermore, doubt has been cast on simplistic renditions of this hypothesis, in that infections per se cannot explain some epidemiologic patterns (e.g., prevalence rates for allergy and asthma are high in some South American countries, where exposures to infection are higher than in some countries with lower rates of asthma 3 ). In addition, not only allergic but also autoimmune and other chronic inflammatory diseases are increasing, 87 a trend that is difficult to explain by the hygiene hypothesis alone, since allergic and autoimmune diseases are associated with competing immunologic phenotypes.
Socio-economic status
Children of parents with lower socio-economic status have greater morbidity from asthma, 88 – 92 but findings with respect to the prevalence of asthma are mixed. 93 – 97 Such results may depend both on how socio-economic status is measured and on the specific outcome examined. Some studies have reported associations of lower socio-economic status with greater airway obstruction and symptoms but not with a diagnosis of asthma. 91 , 92 Whether socio-economic status is as relevant to the incidence of allergy and asthma as it is to the expression, severity and management of these diseases re mains unclear. Parental stress has also been prospectively associated with wheezing in infancy, 46 and family difficulties have been linked to asthma. 48 , 98 Children whose caregivers report high levels of stress and who have difficulties parenting are at greatest risk for asthma. 99
Antibiotics and infections
The use of antibiotics has been associated with early wheezing and asthma in several studies, 47 , 100 , 101 One suggested mechanism for this association is immunologic stimulation through changes in the bowel flora, but Kummeling and associates 100 found no coincident increase in eczema or atopy, despite increased wheezing rates, which would argue against this mechanism. Greater antibiotic use might also represent a surrogate marker for a higher numbers of infections (perhaps viral) in early life.
Viral infections of the lower respiratory tract affect early childhood wheezing. Whether lower respiratory tract infection promotes sensitization to aeroallergens causing persistent asthma is controversial: childhood viral infections might be pathogenic in some children but protective in others. 102 – 106 Infants of mothers with allergy or asthma have a relatively persistent maturational defect in Th1 cytokine synthesis in the first year of life, which may play a role in the development of persistent or severe viral infections. 107 Severe viral infection of the lower respiratory tract in genetically susceptible infants who are already sensitized to inhalant allergens may lead to deviation toward Th2 responses promoting asthma. It is unclear whether these effects of lower respiratory tract infection are virus-specific (e.g., respiratory syncytial virus, rhinovirus) or whether synergistic exposures to allergens can induce asthma even in individuals who are not genetically susceptible. Interactions of genes with environmental exposures (including allergens, air pollution, environmental tobacco smoke and diet) modulate the host response to infections. 108 , 109 It remains controversial whether the occurrence or timing of childhood infection is pathogenic or protective for the development and long-term outcome of asthma and allergy and of nonallergic wheeze phenotypes. This controversy relates in part to small sample size, cross-sectional analysis, lack of precise case definition and incomplete microbial assessment in studies of this phenomenon. 110 , 111
Respiratory infections in early childhood are associated with early wheezing, 109 but it is unclear whether infection alone has a role in the development of persistent asthma. Repeated lower respiratory tract infection may affect infants who are already at risk for asthma because of family history or atopy. 63 , 112 Severe infection with certain viruses such as respiratory syncytial virus 106 and rhinovirus 113 may play a role in persistent wheezing, although other studies have suggested no effect. 114 Considered as a proxy for viral infections, daycare attendance is associated with greater incidence of early wheeze but lower incidence of persistent wheeze. 115
Allergic sensitization
Total serum immunoglobulin E level, a surrogate for allergen sensitivity, has been associated with the incidence of asthma. 116 High levels of immunoglobulin E at birth were associated with greater incidence of both atopy 117 – 119 and aeroallergen sensitivity but not necessarily asthma. However, sensitization to aeroallergens, particularly house dust mite, cat and cockroach allergens, is well documented as being associated with asthma.
Immune responses in the developing infant and young child may affect the development of asthma. For example, impairment in interferon γ production at 3 months was associated with a greater risk of wheeze. 115 Immaturity in neonatal immune responses may promote the persistence of the Th2 immune phenotype and development of atopy, 120 but an association with persistent asthma is as yet unproven. More recent work has focused on the role of the innate immune system in handling and presentation of antigens and suggests that polymorphisms in Toll-like receptors 121 , 122 may play a greater role than previously recognized in the development of the skewed immune responses associated with persistent asthma.
Exposure to environmental tobacco smoke
Postnatal exposure to environmental tobacco smoke, especially from maternal smoking, has been consistently associated with respiratory symptoms of wheezing. 22 , 26 , 56 Exposure to environmental tobacco smoke also consistently worsens asthma symptoms and is a risk factor for severe asthma. 123 , 124

Exposure to animals
Although several studies have demonstrated a lower risk of development of atopy and asthma with exposure to farm animals in early life, the findings of studies of the influence of exposure to domestic cats and dogs have been inconsistent. 125 , 126 In some studies, exposure to cats was associated with a greater risk of allergic sensitization, 127 whereas other studies showed a lower risk. 128 , 129 Exposure to dogs may be protective not only against the development of specific sensitization to dog allergen 127 , 128 but also against other sensitization (e.g., to house dust mites) and asthma. Other studies of exposure to dogs have suggested that protection against wheezing may be mediated by high levels of endotoxin. 130
Gene-by-environment interactions
The effects of gene-by-environment interactions in asthma are complex. In some cases the genes code for enzymes that detoxify inhaled agents (e.g., glutathione transferase genes and environmental pollution), whereas in other cases, the exposures may have a more direct effect on gene expression via epigenetic mechanisms, such as DNA methylation or histone modification. Epigenetic modification of DNA is believed to be responsible for the phenotypic differences that develop over time between monozygotic twins. 131 It has been suggested that it is principally through epigenetic modification of DNA that lifestyle and chemical exposures affect susceptibility to diseases. 132 Nutrition and diet (e.g., folic acid, vitamin B 12 ), smoking, exposure to microbial products, maternal stress and maternal care are potential factors influencing fetal genetic expression, and a further window for epigenetic modification in early life may allow environmental factors to modify a child’s genome with the potential to cause or prolong allergy and asthma. Further work is needed to verify and understand these risks.
Sex and gender
Sex affects the development of asthma in a time-dependent manner. Until age 13–14 years, the incidence and prevalence of asthma are greater among boys than among girls. 133 – 142 Studies through puberty 139 , 143 – 155 have shown a greater incidence of asthma among adolescent and young adult females 133 – 135 , 156 , 157 and a greater proportion of males with remission of asthma. 136 – 140 Before age 12, boys have more severe asthma than girls, 142 with higher rates of admission to hospital. 158 – 165 In contrast, adult females have more severe asthma than males, with more hospital admissions, 161 , 166 , 167 slower improvement, 120 longer hospital stays 161 and higher rates of readmission. 168 Most authors have attributed these changes in prevalence and severity to events of puberty, 140 , 141 although mechanisms for differences between the sexes have not been established.
In childhood, airway hyperresponsiveness is more common and more severe among males; 169 however, airway hyperresponsiveness increases in females during adolescence, 170 , 171 such that by adulthood it is both more common and more severe among adult women. 154 , 155 , 172 – 174 Similar findings have been reported from studies of atopy, which is more common in males before age 13; 175 during adolescence, the rate of new-onset atopy is higher among females, 176 , 177 so that by young adulthood the prevalence of atopy is almost equal.
The influence of some environmental risk factors such as allergens may be modified by sex. In one study of adults, 18% of women with asthma, but only 2.3% of men with asthma, had normal results on common tests related to atopy (negative skin prick tests, immunoglobulin E < 100 IU/mL and eosinophilia < 5%), 178 which suggested different disease mechanisms between the sexes. Interactions have been found between maternal and paternal history of atopy, breastfeeding and sex of the child in terms of the risk of asthma and atopy. 71 Finally, the influence of obesity on the development of asthma is greater among women than among men and has not been shown to be influenced by caloric intake or physical activity. 179 , 180 Some have suggested that the relation between obesity and asthma may be causal, given the consistency, temporal association and dose–response relationships reported in the epidemiologic literature, but the mechanisms remain to be elucidated. 181
Adult-onset asthma
Asthma in adults may have persisted from childhood, may have occurred as a relapse of earlier childhood asthma (whether or not recalled by the individual) or may be true adult-onset asthma with no symptoms in earlier life. 182 – 184 New-onset asthma in adulthood may have environmental (especially occupational) causes with or without allergen sensitization. 185 – 187 Although adult asthma may develop in relation to specific drug treatments (e.g., β-blockers, nonsteroidal anti-inflammatory drugs) or, in women, the use of hormone replacement therapy, 188 occupational exposure to sensitizing agents or irritants is more common.
Occupational asthma
Asthma related to workplace exposures has been documented in many occupational settings. Commonly associated occupations and exposures include car painting (isocyanates), hairdressing (various chemicals), domestic and commercial cleaning (cleaning solutions), health care professions (latex) and baking (flour dust), among many others. 189
The relation between exposure to substances in the work-place and new-onset adult asthma was explored among 6837 participants with no previously reported asthma symptoms in phase I of the European Community Respiratory Health Study. 187 Exposure to substances known to cause occupational asthma was associated with a higher risk of asthma overall (relative risk [RR] 1.6, 95% confidence interval [CI] 1.1–2.3) and of asthma defined by airway hyperresponsiveness (RR 2.4, 95% CI 1.3–4.6). Of common occupations, nursing was associated with the highest risk of occupational asthma (RR 2.2, 95% CI 1.3–4.0, p = 0.007), whereas exposure to an acute inhalation event, such as fire, mixing of cleaning agents or a chemical spill, was associated with an even higher risk (RR 3.3, 95% CI 1.0–11.1, p = 0.05). The population attributable risk of occupational exposure for adult asthma in that study ranged from 10% to 25%.
Other risk factors for adult asthma
Smoking tobacco 190 or marijuana 191 , 192 may give rise to symptoms suggesting asthma, although symptoms of cough and sputum production, suggesting chronic bronchitis, are more common. As in childhood, the differential diagnosis should include other forms of airway inflammation and other causes of intermittent dyspnea and wheezing, such as cardiac failure. However, new-onset asthma can occur at any age, without prior illness or concomitant disease. Atopy as a risk factor for asthma is less common with increasing age, 193 but occasionally it is the dominant trigger. Air pollution may affect adult asthma, but more often it is a factor worsening pre-existing asthma rather than a cause of incident asthma. 194 – 196
Conclusions
Many cross-sectional studies have confirmed increases in the incidence and prevalence of asthma over the past 2 to 3 decades, but much remains unknown as to the fundamental immunologic, genetic and environmental mechanisms underlying the development of this condition and its increased expression, especially in the developed world. Nonetheless, some risk factors have now been clearly and consistently identified. For instance, avoidance of maternal smoking in pregnancy and in the early postpartum period can be strongly encouraged, as can avoidance of known occupational sensitizers. In contrast, previous advice to avoid animals and to breastfeed as long as possible to reduce the risks of asthma has been challenged by more recent studies. It is likely that detailed studies of gene-by-environment interactions and of epigenetics will eventually untangle the inconsistencies among the many putative exposures and outcomes. Although there are indications that the increase in asthma has reached a plateau, at least in countries with the highest prevalence rates, much of the epidemiology and many of the risk factors for asthma remain to be adequately explained. Reduction in risk, and perhaps even true primary prevention of asthma, remains elusive but is a key goal of asthma management.
The prevalence of asthma varies widely around the world, probably because of gene-by-environment interactions.
Prenatal risk factors for asthma may include maternal smoking, diet and nutrition, stress, use of antibiotics and delivery by cesarean section.
Childhood risk factors for asthma may include allergic sensitization, environmental tobacco smoke, exposure to animals, breastfeeding, decreased lung function in infancy, family size and structure, socio-economic status, antibiotics and infections, and sex and gender.
Occupational exposures constitute a common risk factor for adult asthma.
This article is the first in a 7-part case study series that was developed as a knowledge translation initiative of the Canadian Thoracic Society Asthma Committee. The series aims to educate and inform primary care providers and nonrespiratory specialists about the diagnosis and management of asthma. The key messages presented in the cases are not clinical practice guidelines but are based on a review of the most recent scientific evidence available. Financial support for the publication of this series has been provided, in part, by the Canadian Thoracic Society.
Acknowledgement
The authors thank Dr. Peter Paré, iCapture Centre, University of British Columbia, for his invaluable assistance in editing and contributing to the genetics sections of this manuscript.
This article has been peer reviewed.
Competing interests: Piush Mandhane has received speaker’s honoraria from Merck Canada and the Edmonton Thoracic Society. None declared for Padmaja Subbarao and Malcolm Sears.
Contributors: All authors contributed to the development and editing of the publication, and all approved the final version submitted for publication.
Funding: The Canadian Thoracic Society has received funding to facilitate the knowledge translation activities of the CTS Asthma Committee from AstraZeneca Canada, GlaxoSmithKline Inc., Merck Frosst Canada and Novartis Pharmaceuticals. None of the sponsors played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the key messages presented in the case studies.
- 1. Subbarao P, Becker A, Brook JR, et al. CHILD Study Investigators. Epidemiology of asthma: risk factors for development. Expert Rev Clin Immunol. 2009;5:77–95. doi: 10.1586/1744666X.5.1.77. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–32. [ PubMed ] [ Google Scholar ]
- 4. Janson C, Anto J, Burney P, et al. The European Community Respiratory Health Survey: What are the main results so far? European Community Respiratory Health Survey II. Eur Respir J. 2001;18:598–611. doi: 10.1183/09031936.01.00205801. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Asher MI, Montefort S, Bjorksten B, et al. ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Zock JP, Heinrich J, Jarvis D, et al. Distribution and determinants of house dust mite allergens in Europe: the European Community Respiratory Health Survey II. J Allergy Clin Immunol. 2006;118:682–90. doi: 10.1016/j.jaci.2006.04.060. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Cohen RT, Canino GJ, Bird HR, et al. Area of residence, birthplace, and asthma in Puerto Rican children. Chest. 2007;131:1331–8. doi: 10.1378/chest.06-1917. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. von Mutius E, Martinez FD, Fritzsch C, et al. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med. 1994;149:358–64. doi: 10.1164/ajrccm.149.2.8306030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Wang HY, Wong GW, Chen YZ, et al. Prevalence of asthma among Chinese adolescents living in Canada and in China. CMAJ. 2008;179:1133–42. doi: 10.1503/cmaj.071797. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Anto JM, Sunyer J, Rodriguez-Roisin R, et al. Community outbreaks of asthma associated with inhalation of soybean dust. Toxicoepidemiological Committee. N Engl J Med. 1989;320:1097–102. doi: 10.1056/NEJM198904273201701. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Johnston NW, Sears MR. Asthma exacerbations. 1: Epidemiology. Thorax. 2006;61:722–8. doi: 10.1136/thx.2005.045161. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol. 2007;120:526–9. doi: 10.1016/j.jaci.2007.05.047. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Burke W, Fesinmeyer M, Reed K, et al. Family history as a predictor of asthma risk. Am J Prev Med. 2003;24:160–9. doi: 10.1016/s0749-3797(02)00589-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Willemsen G, van Beijsterveldt TCEM, van Baal CGCM, et al. Heritability of self-reported asthma and allergy: a study in adult Dutch twins, siblings and parents. Twin Res Hum Genet. 2008;11:132–42. doi: 10.1375/twin.11.2.132. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Holberg CJ, Elston RC, Halonen M, et al. Segregation analysis of physician-diagnosed asthma in Hispanic and non-Hispanic white families. A recessive component? Am J Respir Crit Care Med. 1996;154:144–50. doi: 10.1164/ajrccm.154.1.8680670. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Lawrence S, Beasley R, Doull I, et al. Genetic analysis of atopy and asthma as quantitative traits and ordered polychotomies. Ann Hum Genet. 1994;58:359–68. doi: 10.1111/j.1469-1809.1994.tb00732.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–3. doi: 10.1038/nature06014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Galanter J, Choudhry S, Eng C, et al. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med. 2008;177:1194–200. doi: 10.1164/rccm.200711-1644OC. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Tavendale R, Macgregor DF, Mukhopadhyay, et al. A polymorphism controlling ORMDL3 expression is associated with asthma that is poorly controlled by current medications. J Allergy Clin Immunol. 2008;121:860–3. doi: 10.1016/j.jaci.2008.01.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Stein RT, Holberg CJ, Sherrill D, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s Respiratory Study. Am J Epidemiol. 1999;149:1030–7. doi: 10.1093/oxfordjournals.aje.a009748. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Lewis S, Richards D, Bynner J, et al. Prospective study of risk factors for early and persistent wheezing in childhood. Eur Respir J. 1995;8:349–56. doi: 10.1183/09031936.95.08030349. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Lau S, Nickel R, Niggemann B, et al. The development of childhood asthma: lessons from the German Multicentre Allergy Study (MAS) Paediatr Respir Rev. 2002;3:265–72. doi: 10.1016/s1526-0542(02)00189-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Tariq SM, Hakim EA, Matthews SM, et al. Influence of smoking on asthmatic symptoms and allergen sensitisation in early childhood. Postgrad Med J. 2000;76:694–9. doi: 10.1136/pmj.76.901.694. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Dezateux C, Stocks J, Dundas I, et al. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999;159:403–10. doi: 10.1164/ajrccm.159.2.9712029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Lødrup Carlsen KC. The Environment and Childhood Asthma (ECA) Study in Oslo: ECA-1 and ECA-2. Pediatr Allergy Immunol. 2002;13(Suppl 15):29–31. doi: 10.1034/j.1399-3038.13.s.15.2.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Macaubas C, de Klerk NH, Holt BJ, et al. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 2003;362:1192–7. doi: 10.1016/s0140-6736(03)14542-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Frey U, Kuehni C, Roiha H, et al. Maternal atopic disease modifies effects of pre-natal risk factors on exhaled nitric oxide in infants. Am J Respir Crit Care Med. 2004;170:260–5. doi: 10.1164/rccm.200307-1002OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Willers SM, Devereux G, Craig LC, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62:773–9. doi: 10.1136/thx.2006.074187. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Romieu I, Torrent M, Garcia-Esteban R, et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin Exp Allergy. 2007;37:518–25. doi: 10.1111/j.1365-2222.2007.02685.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Mihrshahi S, Ampon R, Webb K, et al. The association between infant feeding practices and subsequent atopy among children with a family history of asthma. Clin Exp Allergy. 2007;37:671–9. doi: 10.1111/j.1365-2222.2007.02696.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Litonjua AA, Rifas-Shiman SL, Ly NP, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–11. doi: 10.1093/ajcn/84.4.903. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Devereux G, Turner SW, Craig LC, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174:499–507. doi: 10.1164/rccm.200512-1946OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121–8. doi: 10.1164/rccm.200402-220OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child [review] Cochrane Database Syst Rev. 2006;(3):CD000133. doi: 10.1002/14651858.CD000133.pub2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Fälth-Magnusson K, Kjellman NI. Development of atopic disease in babies whose mothers were receiving exclusion diet during pregnancy — a randomized study. J Allergy Clin Immunol. 1987;80:868–75. doi: 10.1016/s0091-6749(87)80279-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Falth-Magnusson K, Kjellman NI. Allergy prevention by maternal elimination diet during late pregnancy — a 5-year follow-up of a randomized study. J Allergy Clin Immunol. 1992;89:709–13. doi: 10.1016/0091-6749(92)90378-f. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Lilja G, Dannaeus A, Falth-Magnusson K, et al. Immune response of the atopic woman and foetus: effects of high- and low-dose food allergen intake during late pregnancy. Clin Allergy. 1988;18:131–42. doi: 10.1111/j.1365-2222.1988.tb02852.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9. doi: 10.1093/ajcn/85.3.853. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Wright RJ, Finn P, Contreras JP, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–7. doi: 10.1016/j.jaci.2004.03.032. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–9. doi: 10.1097/00130832-200502000-00006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Lin YC, Wen HJ, Lee YL, et al. Are maternal psychosocial factors associated with cord immunoglobulin E in addition to family atopic history and mother immunoglobulin E? Clin Exp Allergy. 2004;34:548–54. doi: 10.1111/j.1365-2222.2004.1928.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Wright RJ, Cohen S, Carey V, et al. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study. Am J Respir Crit Care Med. 2002;165:358–65. doi: 10.1164/ajrccm.165.3.2102016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007;131:1753–9. doi: 10.1378/chest.06-3008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Mrazek DA, Klinnert M, Mrazek PJ, et al. Prediction of early-onset asthma in genetically at-risk children. Pediatr Pulmonol. 1999;27:85–94. doi: 10.1002/(sici)1099-0496(199902)27:2<85::aid-ppul4>3.0.co;2-b. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Jedrychowski W, Galas A, Whyatt R, et al. The prenatal use of antibiotics and the development of allergic disease in one year old infants. A preliminary study. Int J Occup Med Environ Health. 2006;19:70–6. doi: 10.2478/v10001-006-0010-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Benn CS, Thorsen P, Jensen JS, et al. Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of antiasthma medication in early childhood. J Allergy Clin Immunol. 2002;110:72–7. doi: 10.1067/mai.2002.125833. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. McKeever TM, Lewis SA, Smith C, et al. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am J Respir Crit Care Med. 2002;166:827–32. doi: 10.1164/rccm.200202-158OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Nafstad P, Magnus P, Jaakkola JJ. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. J Allergy Clin Immunol. 2000;106:867–73. doi: 10.1067/mai.2000.110558. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Annesi-Maesano I, Moreau D, Strachan D. In utero and perinatal complications preceding asthma. Allergy. 2001;56:491–7. doi: 10.1034/j.1398-9995.2001.056006491.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Lewis S, Butland B, Strachan D, et al. Study of the aetiology of wheezing illness at age 16 in two national British birth cohorts. Thorax. 1996;51:670–6. doi: 10.1136/thx.51.7.670. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Nafstad P, Samuelsen SO, Irgens LM, et al. Pregnancy complications and the risk of asthma among Norwegians born between 1967 and 1993. Eur J Epidemiol. 2003;18:755–61. doi: 10.1023/a:1025395405101. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Stick SM, Burton PR, Gurrin L, et al. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348:1060–4. doi: 10.1016/s0140-6736(96)04446-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Kero J, Gissler M, Gronlund MM, et al. Mode of delivery and asthma — is there a connection? Pediatr Res. 2002;52:6–11. doi: 10.1203/00006450-200207000-00004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Xu B, Pekkanen J, Hartikainen AL, et al. Caesarean section and risk of asthma and allergy in adulthood. J Allergy Clin Immunol. 2001;107:732–3. doi: 10.1067/mai.2001.113048. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Bager P, Melbye M, Rostgaard K, et al. Mode of delivery and risk of allergic rhinitis and asthma. J Allergy Clin Immunol. 2003;111:51–6. doi: 10.1067/mai.2003.34. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Lowe LA, Simpson A, Woodcock A, et al. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–7. doi: 10.1164/rccm.200406-695OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Young S, Arnott J, O’Keeffe PT, et al. The association between early life lung function and wheezing during the first 2 yrs of life. Eur Respir J. 2000;15:151–7. doi: 10.1183/09031936.00.15115100. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Bergmann RL, Diepgen TL, Kuss O, et al. Breastfeeding duration is a risk factor for atopic eczema. Clin Exp Allergy. 2002;32:205–9. doi: 10.1046/j.1365-2222.2002.01274.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Dell S, To T. Breastfeeding and asthma in young children: findings from a population-based study. Arch Pediatr Adolesc Med. 2001;155:1261–5. doi: 10.1001/archpedi.155.11.1261. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Oddy WH. Breastfeeding and asthma in children: findings from a West Australian study. Breastfeed Rev. 2000;8:5–11. [ PubMed ] [ Google Scholar ]
- 67. Sears MR, Greene JM, Willan AR, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360:901–7. doi: 10.1016/S0140-6736(02)11025-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Wright AL, Holberg CJ, Taussig LM, et al. Maternal asthma status alters relation of infant feeding to asthma in childhood. Adv Exp Med Biol. 2000;478:131–7. doi: 10.1007/0-306-46830-1_11. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001;139:261–6. doi: 10.1067/mpd.2001.117006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Kull I, Almqvist C, Lilja G, et al. Breast-feeding reduces the risk of asthma during the first 4 years of life. J Allergy Clin Immunol. 2004;114:755–60. doi: 10.1016/j.jaci.2004.07.036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Mandhane PJ, Greene JM, Cowan JO, et al. Sex differences in factors associated with childhood- and adolescent-onset wheeze. Am J Respir Crit Care Med. 2005;172:45–54. doi: 10.1164/rccm.200412-1738OC. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Businco L, Marchetti F, Pellegrini G, et al. Predictive value of cord blood IgE levels in ‘at-risk’ newborn babies and influence of type of feeding. Clin Allergy. 1983;13:503–8. doi: 10.1111/j.1365-2222.1983.tb02631.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Lovegrove JA, Hampton SM, Morgan JB. The immunological and long-term atopic outcome of infants born to women following a milk-free diet during late pregnancy and lactation: a pilot study. Br J Nutr. 1994;71:223–38. doi: 10.1079/bjn19940129. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Sigurs N, Hattevig G, Kjellman B. Maternal avoidance of eggs, cow’s milk, and fish during lactation: effect on allergic manifestations, skin-prick tests, and specific IgE antibodies in children at age 4 years. Pediatrics. 1992;89:735–9. [ PubMed ] [ Google Scholar ]
- 75. Hattevig G, Sigurs N, Kjellman B. Effects of maternal dietary avoidance during lactation on allergy in children at 10 years of age. Acta Paediatr. 1999;88:7–12. [ PubMed ] [ Google Scholar ]
- 76. Muraro A, Dreborg S, Halken S, et al. Dietary prevention of allergic diseases in infants and small children. Part III: Critical review of published peer-reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol. 2004;15:291–307. doi: 10.1111/j.1399-3038.2004.00127.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Haland G, Carlsen KC, Sandvik L, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–9. doi: 10.1056/NEJMoa052885. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol. 2002;109:189–94. doi: 10.1067/mai.2002.120951. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Clarke JR, Reese A, Silverman M. Bronchial responsiveness and lung function in infants with lower respiratory tract illness over the first six months of life. Arch Dis Child. 1992;67:1454–8. doi: 10.1136/adc.67.12.1454. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Turner SW, Palmer LJ, Rye PJ, et al. The relationship between infant airway function, childhood airway responsiveness, and asthma. Am J Respir Crit Care Med. 2004;169:921–7. doi: 10.1164/rccm.200307-891OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–77. doi: 10.1016/j.jaci.2006.03.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Matricardi PM, Franzinelli F, Franco A, et al. Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol. 1998;101:439–44. doi: 10.1016/s0091-6749(98)70350-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Kinra S, Davey SG, Jeffreys M, et al. Association between sibship size and allergic diseases in the Glasgow Alumni Study. Thorax. 2006;61:48–53. doi: 10.1136/thx.2004.034595. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Goldberg S, Israeli E, Schwartz S, et al. Asthma prevalence, family size, and birth order. Chest. 2007;131:1747–52. doi: 10.1378/chest.06-2818. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Bernsen RM, de Jongste JC, van der Wouden JC. Birth order and sibship size as independent risk factors for asthma, allergy, and eczema. Pediatr Allergy Immunol. 2003;14:464–9. doi: 10.1046/j.0905-6157.2003.00108.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics. 1993;91:56–61. [ PubMed ] [ Google Scholar ]
- 89. Goodman DC, Stukel TA, Chang CH. Trends in pediatric asthma hospitalization rates: regional and socioeconomic differences. Pediatrics. 1998;101:208–13. doi: 10.1542/peds.101.2.208. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Claudio L, Tulton L, Doucette J, et al. Socioeconomic factors and asthma hospitalization rates in New York City. J Asthma. 1999;36:343–50. doi: 10.3109/02770909909068227. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Erzen D, Carriere KC, Dik N, et al. Income level and asthma prevalence and care patterns. Am J Respir Crit Care Med. 1997;155:1060–5. doi: 10.1164/ajrccm.155.3.9116987. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Strachan DP, Anderson HR, Limb ES, et al. A national survey of asthma prevalence, severity, and treatment in Great Britain. Arch Dis Child. 1994;70:174–8. doi: 10.1136/adc.70.3.174. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Litonjua AA, Carey VJ, Weiss ST, et al. Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatr Pulmonol. 1999;28:394–401. doi: 10.1002/(sici)1099-0496(199912)28:6<394::aid-ppul2>3.0.co;2-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. Am J Public Health. 2000;90:428–30. doi: 10.2105/ajph.90.3.428. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Peckham C, Butler N. A national study of asthma in childhood. J Epidemiol Community Health. 1978;32:79–85. doi: 10.1136/jech.32.2.79. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Hamman RF, Halil T, Holland WW. Asthma in schoolchildren. Demographic associations and peak expiratory flow rates compared in children with bronchitis. Br J Prev Soc Med. 1975;29:228–38. doi: 10.1136/jech.29.4.228. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Ernst P, Demissie K, Joseph L, et al. Socioeconomic status and indicators of asthma in children. Am J Respir Crit Care Med. 1995;152:570–5. doi: 10.1164/ajrccm.152.2.7633709. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Klinnert MD, Nelson HS, Price MR, et al. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108:E69. doi: 10.1542/peds.108.4.e69. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Klinnert MD, Mrazek PJ, Mrazek DA. Early asthma onset: the interaction between family stressors and adaptive parenting. Psychiatry. 1994;57:51–61. doi: 10.1080/00332747.1994.11024668. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Kummeling I, Stelma FF, Dagnelie PC, et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2007;119:e225–31. doi: 10.1542/peds.2006-0896. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Alm B, Erdes L, Mollborg P, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics. 2008;121:697–702. doi: 10.1542/peds.2007-1232. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Lemanske RF, Jr, Busse WW. Asthma: factors underlying inception, exacerbation, and disease progression. J Allergy Clin Immunol. 2006;117(2 Suppl Mini-Primer):S456–61. doi: 10.1016/j.jaci.2005.07.006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Arruda LK, Sole D, Baena-Cagnani CE, et al. Risk factors for asthma and atopy. Curr Opin Allergy Clin Immunol. 2005;5:153–9. doi: 10.1097/01.all.0000162308.89857.6c. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Martinez FD. Role of viral infections in the inception of asthma and allergies during childhood: Could they be protective? Thorax. 1994;49:1189–91. doi: 10.1136/thx.49.12.1189. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Martinez FD, Holt PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354(Suppl 2):SII12–5. doi: 10.1016/s0140-6736(99)90437-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Gern JE, Brooks GD, Meyer P, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–8. doi: 10.1016/j.jaci.2005.10.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Friedlander SL, Jackson DJ, Gangnon RE, et al. Viral infections, cytokine dysregulation and the origins of childhood asthma and allergic diseases. Pediatr Infect Dis J. 2005;24(Suppl):S170–6. doi: 10.1097/01.inf.0000187273.47390.01. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Becker A, Watson W, Ferguson A, et al. The Canadian Asthma Primary Prevention Study: outcomes at 2 years of age. J Allergy Clin Immunol. 2004;113:650–6. doi: 10.1016/j.jaci.2004.01.754. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Chan-Yeung M, Ferguson A, Watson W, et al. The Canadian Childhood Asthma Primary Prevention Study: outcomes at 7 years of age. J Allergy Clin Immunol. 2005;116:49–55. doi: 10.1016/j.jaci.2005.03.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Oddy WH, de Klerk NH, Sly PD, et al. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J. 2002;19:899–905. doi: 10.1183/09031936.02.00103602. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Joshi P, Shaw A, Kakakios A, et al. Interferon-gamma levels in nasopharyngeal secretions of infants with respiratory syncytial virus and other respiratory viral infections. Clin Exp Immunol. 2003;131:143–7. doi: 10.1046/j.1365-2249.2003.02039.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Celedón JC, Litonjua AA, Ryan L, et al. Day care attendance, respiratory tract illnesses, wheezing, asthma, and total serum IgE level in early childhood. Arch Pediatr Adolesc Med. 2002;156:241–5. doi: 10.1001/archpedi.156.3.241. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Sears MR, Burrows B, Flannery EM, et al. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–71. doi: 10.1056/NEJM199110103251504. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Tariq SM, Arshad SH, Matthews SM, et al. Elevated cord serum IgE increases the risk of aeroallergen sensitization without increasing respiratory allergic symptoms in early childhood. Clin Exp Allergy. 1999;29:1042–8. doi: 10.1046/j.1365-2222.1999.00594.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Wickman M, Ahlstedt S, Lilja G, et al. Quantification of IgE antibodies simplifies the classification of allergic diseases in 4-year-old children. A report from the prospective birth cohort study – BAMSE. Pediatr Allergy Immunol. 2003;14:441–7. doi: 10.1046/j.0905-6157.2003.00079.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Karmaus W, Arshad H, Mattes J. Does the sibling effect have its origin in utero? Investigating birth order, cord blood immunoglobulin E concentration, and allergic sensitization at age 4 years. Am J Epidemiol. 2001;154:909–15. doi: 10.1093/aje/154.10.909. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Prescott SL, King B, Strong TL, et al. The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy. 2003;58:1187–94. doi: 10.1034/j.1398-9995.2003.00263.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Tulic MK, Fiset PO, Manoukian JJ, et al. Role of Toll-like receptor 4 in protection by bacterial lipopolysaccharide in the nasal mucosa of atopic children but not adults. Lancet. 2004;363:1689–97. doi: 10.1016/S0140-6736(04)16253-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Lauener RP, Birchler T, Adamski J, et al. Expression of CD14 and Toll-like receptor 2 in farmers’ and non-farmers’ children. Lancet. 2002;360:465–6. doi: 10.1016/S0140-6736(02)09641-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Lin RY, Rehman A. Clinical characteristics of adult asthmatics requiring intubation. J Med. 1995;26:261–77. [ PubMed ] [ Google Scholar ]
- 124. James AL, Palmer LJ, Kicic E, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171:109–14. doi: 10.1164/rccm.200402-230OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 125. Simpson A, Custovic A. Pets and the development of allergic sensitization. Curr Allergy Asthma Rep. 2005;5:212–20. doi: 10.1007/s11882-005-0040-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 126. Takkouche B, Gonzalez-Barcala FJ, Etminan M, et al. Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta-analysis. Allergy. 2008;63:857–64. doi: 10.1111/j.1398-9995.2008.01732.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Almqvist C, Egmar AC, Hedlin G, et al. Direct and indirect exposure to pets —risk of sensitization and asthma at 4 years in a birth cohort. Clin Exp Allergy. 2003;33:1190–7. doi: 10.1046/j.1365-2222.2003.01764.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 128. Huss K, Adkinson NF, Jr, Eggleston PA, et al. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. [ DOI ] [ PubMed ] [ Google Scholar ]
- 129. Henriksen AH, Holmen TL, Bjermer L. Sensitization and exposure to pet allergens in asthmatics versus non-asthmatics with allergic rhinitis. Respir Med. 2001;95:122–9. doi: 10.1053/rmed.2000.1004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 130. Campo P, Kalra HK, Levin L, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol. 2006;118:1271–8. doi: 10.1016/j.jaci.2006.08.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Qiu J. Epigenetics: unfinished symphony. Nature. 2006;441:143–5. doi: 10.1038/441143a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. de Marco R, Locatelli F, Sunyer J, et al. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Crawford WA, Beedham CG. The changing demographic pattern in asthma related to sex and age. A study of 13,651 patients on sodium cromoglycate (Intal) Med J Aust. 1976;1:430–4. [ PubMed ] [ Google Scholar ]
- 135. Bjornson CL, Mitchell I. Gender differences in asthma in childhood and adolescence. J Gend Specif Med. 2000;3:57–61. [ PubMed ] [ Google Scholar ]
- 136. de Marco R, Locatelli F, Cerveri I, et al. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110:228–35. doi: 10.1067/mai.2002.125600. [ DOI ] [ PubMed ] [ Google Scholar ]
- 137. Sears MR. Growing up with asthma. BMJ. 1994;309:72–3. doi: 10.1136/bmj.309.6947.72. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. Weiss ST, Sparrow D, O’Connor GT. The interrelationship among allergy, airways responsiveness, and asthma. J Asthma. 1993;30:329–49. doi: 10.3109/02770909309056738. [ DOI ] [ PubMed ] [ Google Scholar ]
- 139. Bronnimann S, Burrows B. A prospective study of the natural history of asthma. Remission and relapse rates. Chest. 1986;90:480–4. doi: 10.1378/chest.90.4.480. [ DOI ] [ PubMed ] [ Google Scholar ]
- 140. Nicolai T, Illi S, Tenborg J, et al. Puberty and prognosis of asthma and bronchial hyper-reactivity. Pediatr Allergy Immunol. 2001;12:142–8. doi: 10.1034/j.1399-3038.2001.0007.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 141. Zannolli R, Morgese G. Does puberty interfere with asthma? Med Hypotheses. 1997;48:27–32. doi: 10.1016/s0306-9877(97)90020-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Meurer JR, George V, Subichin S, et al. Asthma severity among children hospitalized in 1990 and 1995. Arch Pediatr Adolesc Med. 2000;154:143–9. doi: 10.1001/archpedi.154.2.143. [ DOI ] [ PubMed ] [ Google Scholar ]
- 143. Williams H, McNicol KN. Prevalence, natural history, and relationship of wheezy bronchitis and asthma in children. An epidemiological study. BMJ. 1969;4:321–5. doi: 10.1136/bmj.4.5679.321. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Gerritsen J, Koëter GH, Postma DS, et al. Prognosis of asthma from childhood to adulthood. Am Rev Respir Dis. 1989;140:1325–30. doi: 10.1164/ajrccm/140.5.1325. [ DOI ] [ PubMed ] [ Google Scholar ]
- 145. Roche WR. Inflammatory and structural changes in the small airways in bronchial asthma. Am J Respir Crit Care Med. 1998;157:S191–4. doi: 10.1164/ajrccm.157.5.rsaa-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 146. Schwartz J, Weiss ST. Relationship of skin test reactivity to decrements in pulmonary function in children with asthma or frequent wheezing. Am J Respir Crit Care Med. 1995;152:2176–80. doi: 10.1164/ajrccm.152.6.8520794. [ DOI ] [ PubMed ] [ Google Scholar ]
- 147. Selroos O, Pietinalho A, Lofroos AB, et al. Effect of early vs late intervention with inhaled corticosteroids in asthma. Chest. 1995;108:1228–34. doi: 10.1378/chest.108.5.1228. [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Sherrill DL, Martinez FD, Lebowitz MD, et al. Longitudinal effects of passive smoking on pulmonary function in New Zealand children. Am Rev Respir Dis. 1992;145:1136–41. doi: 10.1164/ajrccm/145.5.1136. [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. Sherrill D, Sears MR, Lebowitz MD, et al. The effects of airway hyperresponsiveness, wheezing, and atopy on longitudinal pulmonary function in children: a 6-year follow-up study. Pediatr Pulmonol. 1992;13:78–85. doi: 10.1002/ppul.1950130204. [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Oswald H, Phelan PD, Lanigan A, et al. Childhood asthma and lung function in mid-adult life. Pediatr Pulmonol. 1997;23:14–20. doi: 10.1002/(sici)1099-0496(199701)23:1<14::aid-ppul2>3.0.co;2-p. [ DOI ] [ PubMed ] [ Google Scholar ]
- 151. Kjellman B, Hesselmar B. Prognosis of asthma in children: a cohort study into adulthood. Acta Paediatr. 1994;83:854–61. doi: 10.1111/j.1651-2227.1994.tb13157.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Sears MR, Herbison GP, Holdaway MD, et al. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989;19:419–24. doi: 10.1111/j.1365-2222.1989.tb02408.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 153. Sears MR, Holdaway MD, Flannery EM, et al. Parental and neonatal risk factors for atopy, airway hyper-responsiveness, and asthma. Arch Dis Child. 1996;75:392–8. doi: 10.1136/adc.75.5.392. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 154. Roorda RJ, Gerritsen J, van Aalderen WM, et al. Follow-up of asthma from childhood to adulthood: influence of potential childhood risk factors on the outcome of pulmonary function and bronchial responsiveness in adulthood. J Allergy Clin Immunol. 1994;93:575–84. doi: 10.1016/s0091-6749(94)70069-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 155. Godden DJ, Ross S, Abdalla M, et al. Outcome of wheeze in childhood. Symptoms and pulmonary function 25 years later. Am J Respir Crit Care Med. 1994;149:106–12. doi: 10.1164/ajrccm.149.1.8111567. [ DOI ] [ PubMed ] [ Google Scholar ]
- 156. Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54:1119–38. doi: 10.1136/thx.54.12.1119. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 157. Dodge RR, Burrows B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis. 1980;122:567–75. doi: 10.1164/arrd.1980.122.4.567. [ DOI ] [ PubMed ] [ Google Scholar ]
- 158. Kao CC, See LC, Yan DC, et al. Time trends and seasonal variations in hospital admissions for childhood asthma in Taiwan from 1990 to 1998. Asian Pac J Allergy Immunol. 2001;19:63–8. [ PubMed ] [ Google Scholar ]
- 159. Joseph CL, Havstad SL, Ownby DR, et al. Racial differences in emergency department use persist despite allergist visits and prescriptions filled for antiinflammatory medications. J Allergy Clin Immunol. 1998;101:484–90. doi: 10.1016/S0091-6749(98)70355-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 160. Schaubel D, Johansen H, Mao Y, et al. Risk of preschool asthma: incidence, hospitalization, recurrence, and readmission probability. J Asthma. 1996;33:97–103. doi: 10.3109/02770909609054537. [ DOI ] [ PubMed ] [ Google Scholar ]
- 161. Skobeloff EM, Spivey WH, St Clair SS, et al. The influence of age and sex on asthma admissions. JAMA. 1992;268:3437–40. [ PubMed ] [ Google Scholar ]
- 162. To T, Dick P, Feldman W, et al. A cohort study on childhood asthma admissions and readmissions. Pediatrics. 1996;98:191–5. [ PubMed ] [ Google Scholar ]
- 163. Von Behren J, Kreutzer R, Smith D. Asthma hospitalization trends in California, 1983–1996. J Asthma. 1999;36:575–82. doi: 10.3109/02770909909087294. [ DOI ] [ PubMed ] [ Google Scholar ]
- 164. Wilkins K, Mao Y. Trends in rates of admission to hospital and death from asthma among children and young adults in Canada during the 1980s. CMAJ. 1993;148:185–90. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 165. Hyndman SJ, Williams DR, Merrill SL, et al. Rates of admission to hospital for asthma. BMJ. 1994;308:1596–600. doi: 10.1136/bmj.308.6944.1596. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 166. Trawick DR, Holm C, Wirth J. Influence of gender on rates of hospitalization, hospital course, and hypercapnea in high-risk patients admitted for asthma: a 10-year retrospective study at Yale-New Haven Hospital. Chest. 2001;119:115–9. doi: 10.1378/chest.119.1.115. [ DOI ] [ PubMed ] [ Google Scholar ]
- 167. Chen Y, Stewart P, Johansen H, et al. Sex difference in hospitalization due to asthma in relation to age. J Clin Epidemiol. 2003;56:180–7. doi: 10.1016/s0895-4356(02)00593-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 168. Chen Y, Dales R, Stewart P, et al. Hospital readmissions for asthma in children and young adults in Canada. Pediatr Pulmonol. 2003;36:22–6. doi: 10.1002/ppul.10307. [ DOI ] [ PubMed ] [ Google Scholar ]
- 169. Le Souëf PN, Sears MR, Sherrill D. The effect of size and age of subject on airway responsiveness in children. Am J Respir Crit Care Med. 1995;152:576–9. doi: 10.1164/ajrccm.152.2.7633710. [ DOI ] [ PubMed ] [ Google Scholar ]
- 170. Ernst P, Ghezzo H, Becklake MR. Risk factors for bronchial hyperresponsiveness in late childhood and early adolescence. Eur Respir J. 2002;20:635–9. doi: 10.1183/09031936.02.00962002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 171. Gustafsson PM, Kjellman B. Asthma from childhood to adulthood: course and outcome of lung function. Respir Med. 2000;94:466–74. doi: 10.1053/rmed.1999.0763. [ DOI ] [ PubMed ] [ Google Scholar ]
- 172. Tashkin DP, Altose MD, Bleecker ER, et al. The Lung Health Study: airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation. The Lung Health Study Research Group. Am Rev Respir Dis. 1992;145:301–10. doi: 10.1164/ajrccm/145.2_Pt_1.301. [ DOI ] [ PubMed ] [ Google Scholar ]
- 173. Kanner RE, Connett JE, Altose MD, et al. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med. 1994;150:956–61. doi: 10.1164/ajrccm.150.4.7921469. [ DOI ] [ PubMed ] [ Google Scholar ]
- 174. Leynaert B, Bousquet J, Henry C, et al. Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am J Respir Crit Care Med. 1997;156:1413–20. doi: 10.1164/ajrccm.156.5.9701060. [ DOI ] [ PubMed ] [ Google Scholar ]
- 175. Sears MR, Burrows B, Flannery EM, et al. Atopy in childhood. I. Gender and allergen related risks for development of hay fever and asthma. Clin Exp Allergy. 1993;23:941–8. doi: 10.1111/j.1365-2222.1993.tb00279.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 176. Barbee RA, Kaltenborn W, Lebowitz MD, et al. Longitudinal changes in allergen skin test reactivity in a community population sample. J Allergy Clin Immunol. 1987;79:16–24. doi: 10.1016/s0091-6749(87)80010-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 177. Cline MG, Burrows B. Distribution of allergy in a population sample residing in Tucson, Arizona. Thorax. 1989;44:425–31. doi: 10.1136/thx.44.5.425. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 178. Oryszczyn MP, Bouzigon E, Maccario J, et al. Interrelationships of quantitative asthma-related phenotypes in the Epidemiological Study on the Genetics and Environment of Asthma, Bronchial Hyperresponsiveness, and Atopy. J Allergy Clin Immunol. 2007;119:57–63. doi: 10.1016/j.jaci.2006.09.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 179. Camargo CA, Jr, Weiss ST, Zhang S, et al. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–8. doi: 10.1001/archinte.159.21.2582. [ DOI ] [ PubMed ] [ Google Scholar ]
- 180. Hancox RJ, Milne BJ, Poulton R, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171:440–5. doi: 10.1164/rccm.200405-623OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 181. Weiss ST. Obesity: insight into the origins of asthma. Nat Immunol. 2005;6:537–9. doi: 10.1038/ni0605-537. [ DOI ] [ PubMed ] [ Google Scholar ]
- 182. Bauer BA, Reed CE, Yunginger JW, et al. Incidence and outcomes of asthma in the elderly. A population-based study in Rochester, Minnesota. Chest. 1997;111:303–10. doi: 10.1378/chest.111.2.303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 183. Butland BK, Strachan DP. Asthma onset and relapse in adult life: the British 1958 birth cohort study. Ann Allergy Asthma Immunol. 2007;98:337–43. doi: 10.1016/S1081-1206(10)60879-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 184. Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–13. doi: 10.1016/S0140-6736(06)69290-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 185. Eagan TM, Gulsvik A, Eide GE, et al. Occupational airborne exposure and the incidence of respiratory symptoms and asthma. Am J Respir Crit Care Med. 2002;166:933–8. doi: 10.1164/rccm.200203-238OC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 186. Le Moual N, Kennedy SM, Kauffmann F. Occupational exposures and asthma in 14,000 adults from the general population. Am J Epidemiol. 2004;160:1108–16. doi: 10.1093/aje/kwh316. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 187. Kogevinas M, Zock JP, Jarvis D, et al. Exposure to substances in the workplace and new-onset asthma: an international prospective population-based study (ECRHS-II) Lancet. 2007;370:336–41. doi: 10.1016/S0140-6736(07)61164-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 188. Troisi RJ, Speizer FE, Willett WC, et al. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995;152:1183–8. doi: 10.1164/ajrccm.152.4.7551368. [ DOI ] [ PubMed ] [ Google Scholar ]
- 189. Bakerly ND, Moore VC, Vellore AD, et al. Fifteen-year trends in occupational asthma: data from the Shield surveillance scheme. Occup Med (Lond) 2008;58:169–74. doi: 10.1093/occmed/kqn007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 190. Gilliland FD, Islam T, Berhane K, et al. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 2006;174:1094–100. doi: 10.1164/rccm.200605-722OC. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 191. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221–8. doi: 10.1001/archinte.167.3.221. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 192. Taylor DR, Fergusson DM, Milne BJ, et al. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction. 2002;97:1055–61. doi: 10.1046/j.1360-0443.2002.00169.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 193. Jaakkola MS, Ieromnimon A, Jaakkola JJ. Are atopy and specific IgE to mites and molds important for adult asthma? J Allergy Clin Immunol. 2006;117:642–8. doi: 10.1016/j.jaci.2005.11.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 194. McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–58. doi: 10.1056/NEJMoa071535. [ DOI ] [ PubMed ] [ Google Scholar ]
- 195. Chen CH, Xirasagar S, Lin HC. Seasonality in adult asthma admissions, air pollutant levels, and climate: a population-based study. J Asthma. 2006;43:287–92. doi: 10.1080/02770900600622935. [ DOI ] [ PubMed ] [ Google Scholar ]
- 196. Johnston FH, Webby RJ, Pilotto LS, et al. Vegetation fires, particulate air pollution and asthma: a panel study in the Australian monsoon tropics. Int J Environ Health Res. 2006;16:391–404. doi: 10.1080/09603120601093642. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.4 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Asthma articles from across Nature Portfolio
Asthma is a form of bronchial disorder caused by inflammation of the bronchi. It is characterized by spasmodic contraction of airway smooth muscle, difficulty breathing, wheezing and coughing.
Latest Research and Reviews
Improving asthma care in children: revealing needs and bottlenecks through in-depth interviews
- Casper E. W. Gijsen
- Carolien van Rossem
- Edward Dompeling
Fine-tuning levels of filamins a and b as a specific mechanism sustaining Th2 lymphocyte functions
Filamin proteins are degraded by ASB2α E3 ubiquitin ligase and may affect T cell function. Here the authors show that increased levels of Filamin A and Filamin B in Th2 cells reduces allergic inflammation in mouse models through reduced Th2 cell recruitment into inflamed lungs.
- Kilian Maire
- Isabelle Lamsoul
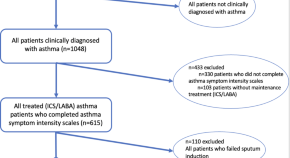
When patient-reported respiratory symptoms shed light on pathophysiology in adult asthma: a cross-sectional study
- Gilles Louis
- Benoit Pétré
- Renaud Louis
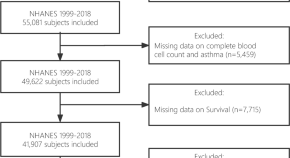
Associations of advanced lung cancer inflammation index with all-cause and respiratory disease mortality in adults with asthma: NHANES, 1999–2018
- Dianliang Zhang

Use and acceptability of an asthma diagnosis clinical decision support system for primary care clinicians: an observational mixed methods study
- Luke Daines
- Hilary Pinnock
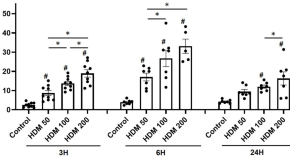
House dust mites stimulate thymic stromal lymphopoietin production in human bronchial epithelial cells and promote airway remodeling through activation of PAR2 and ERK signaling pathway
- Yi-An Hsieh
- Yi-Han Hsiao
- Kang-Cheng Su
News and Comment
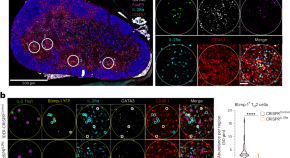
Spatial cytokine microniches direct T helper cell pathways that drive allergic asthma
Using temporal and spatial tracking of allergen-specific T cells, we describe the gene regulatory networks that drive T helper 2 cells (T H 2 cells) to develop in response to allergens. Spatial microniches of IL-2 in lymph nodes supported the earliest T H 2 cells, demonstrating lymph node localization is a driver of T H 2 cell initiation.
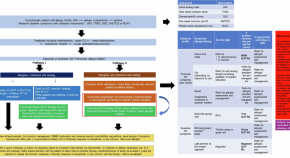
Improving allergy management and treatment: a proposed algorithm and curriculum for prescribing allergen immunotherapy in the primary care setting
Allergic rhinitis (AR), a condition characterized by sensitivity to allergens leading to poor quality of life, including disrupted sleep, reduced vitality, lowered mood, changes in blood pressure limited frustration tolerance, impaired focus, decreased performance in academic and professional settings, and millions of missed work and school days every year. Approximately 20–40% of individuals in the United States are affected by AR, which carries notable clinical and financial burdens. Interestingly, there is a strong link between AR and asthma to the extent that countries with a high prevalence of rhinitis have asthma rates ranging from 10% to 25%. Research has indicated that Allergen Immunotherapy (AIT) is associated with improved AR symptoms, a potential to resolve the AR over time, a decreased likelihood of asthma exacerbations and incidence of pneumonia in individuals with concurrent asthma, which are advantages that persist for years even after the cessation of treatment. Although patients presenting with allergies are first seen and treated in the primary care setting, gaps in training and the lack of available guidance for primary care practitioners have significantly impacted the quality of care for these patients with persistent AR symptoms, resulting in inefficient use of healthcare resources. To complicate matters, there is an insufficiency of allergists and immunologists, impacting the capacity to provide next-level care to the number of AR patients who could benefit from AIT. Hence, there is a critical need to equip primary care providers with educational experiences on essential concepts related to immune responses in allergies and asthma, recognizing the significance of the common airway in treating these entities and familiarization with the scientific evidence supporting various options for AIT. The development and implementation of medical education and algorithms designed to assess diverse patients’ symptoms, pharmacotherapy approaches, and situations where AIT can be initiated or sustained are warranted. The present commentary proposes a workflow model of the critical steps for managing and treating mild to moderate respiratory allergies via AIT in primary care settings. In addition, the initial development of medical education programs to minimize the burden on allergy-specialized care while, importantly, actively improving patient outcomes will be discussed.
- Giseth Bustos
- Marcos A. Sanchez-Gonzalez
- Alan Kaplan

Early life antibiotics have lasting effects on the lung epithelium
Transient depletion of the gut microbiome by antibiotics in early life reduces systemic levels of the metabolite indole-3-propionic acid, which causes long-lasting mitochondrial damage to lung epithelial cells and increases susceptibility to airway inflammation in adult mice.

CAR T cells take to the airways
CAR T cell therapy alleviates type 2 airway inflammation in mouse models of asthma.
- Yvonne Bordon

CAR T cells put the brakes on asthma
CAR T cell technology is being extended beyond the treatment of cancer. New data show that it might also treat allergic asthma, with a single infusion sufficient to prevent pathology for over a year in mice.
- Bart N. Lambrecht
- Hamida Hammad

How to make Asthma Right Care ‘easy’ in primary care: learnings from the 2023 Asthma Right Care Summit
- Siân Williams
- Jaime Correia de Sousa
- Darush Attar-Zadeh
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

COMMENTS
Oct 1, 2018 · Asthma is a disorder characterized by chronic airway inflammation, airway hypersensitivity to a variety of stimuli, and airway obstruction. It is at least partially reversible, either ...
Asthma is a chronic inflammatory disorder arising from not fully understood heterogenic gene-environment interactions. It features variable airway obstruction and bronchial hyperresponsiveness. Clinically, asthmatics exhibit recurrent episodes of wheeze, cough, chest tightness, and shortness of brea …
May 3, 2024 · Asthma is a prevalent chronic inflammatory respiratory condition affecting millions of people worldwide and presents substantial challenges in both diagnosis and management. This respiratory condition is characterized by inflammation of the airways, causing intermittent airflow obstruction and bronchial hyperresponsiveness. The hallmark asthma symptoms include coughing, wheezing, and shortness ...
In this review, we discuss recent publications on asthma and review the studies that have reported on the different aspects of the prevalence, risk factors and prevention, mechanisms, diagnosis, and treatment of asthma. Many risk and protective factors and molecular mechanisms are involved in the de …
Oct 5, 2011 · Asthma is a lifelong condition with varying degrees of severity and susceptibility to symptom control. Recent studies have examined the effects of individual genus, species, and strains of ...
Jul 18, 2017 · Asthma is characterized by variable airway obstruction, airway hyperresponsiveness, and airway inflammation. Management of persistent asthma requires avoidance of aggravating environmental factors, use of short-acting β2-agonists for rapid relief of symptoms, and daily use of inhaled corticosteroids …
Asthma is one of the most common chronic conditions affecting both children and adults, yet much remains to be learned of its etiology. This paper evolved from the extensive literature review undertaken as part of a proposal for a longitudinal birth cohort study to examine risk factors for the development of allergy and asthma in early childhood.
2 days ago · Asthma is a form of bronchial disorder caused by inflammation of the bronchi. It is characterized by spasmodic contraction of airway smooth muscle, difficulty breathing, wheezing and coughing ...
Jul 2, 2023 · Moreover, based on the Severe Asthma Research Program (SARP) multiple asthma phenotypes focusing on the spectrum of asthma severity have been proposed (early-onset allergic asthma, late-onset severe asthma, and severe asthma with chronic obstructive pulmonary disease characteristics) (Citation 12, Citation 13).
Mar 19, 2021 · Asthma is a heterogeneous disease that in children is often allergen-driven with a type 2 inflammation. Sublingual immunotherapy represents an important progress in the use of personalized ...