- Science, Tech, Math ›
- Chemistry ›
- Projects & Experiments ›

Simple Candy Osmosis Experiment
Demonstrate Osmosis Using Gummy Bears
- Projects & Experiments
- Chemical Laws
- Periodic Table
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Osmosis is the diffusion of water across a semipermeable membrane. The water moves from an area of higher to lower solvent concentration (an area of lower to higher solute concentration). It's an important passive transport process in living organisms, with applications to chemistry and other sciences. You don't need fancy lab equipment to observe osmosis. You can experiment with the phenomenon using gummy bears and water. Here's what you do:
Osmosis Experiment Materials
Basically, all you need for this chemistry project are colored candies and water:
- Gummy bear candies (or other gummy candy)
- Plate or shallow bowl
The gelatin of the gummy candies acts as a semipermeable membrane . Water can enter the candy, but it's much harder for sugar and coloring to leave exit it.
What You Do
It's easy! Simply place one or more of the candies in the dish and pour in some water. Over time, water will enter the candies, swelling them. Compare the size and "squishiness" of these candies with how they looked before. Notice the colors of the gummy bears starts to appear lighter. This is because the pigment molecules (solute molecules) are being diluted by the water (solvent molecules) as the process progresses.
What do you think would happen if you used a different solvent, such as milk or honey, that already contains some solute molecules? Make a prediction, then try it and see.
How do you think osmosis in a gelatin dessert compares with osmosis in candy? Again, make a prediction and then test it!
- How to Do Chromatography with Candy and Coffee Filters
- Candy Chemistry Projects
- Exploding Mentos Drink Experiment
- How to Perform the Dancing Gummi Bear Demonstration
- How to Get Fluoride Out of Water
- Honeycomb Chemistry Candy Recipe
- Middle School Science Fair Project Ideas
- Take Your Volcano Science Project to the Next Level
- Sugar Crystal Growing Problems
- Experiment to See How Much Sugar Is in a Soda
- Make Candy Glass Icicle Decorations
- How to Make Rock Candy
- The Dancing Raisin Experiment
- How to Make a DNA Model out of Candy
- Why Is the Sky Blue?
- Make Potassium Chlorate from Bleach and Salt Substitute

6 EASY ways to model OSMOSIS

Teaching osmosis?
Osmosis is one of my favorite topics to teach during the cells unit because it’s so easily visualized. There are quite a few ways to easily see osmosis in action! Here’s a round-up of six labs and the pros and cons of each:
1. EGG OSMOSIS
The “naked egg” lab is very popular in biology. Students really get a kick out of seeing the eggshell dissolve! In this lab, students dissolve the shell of an egg by soaking it in vinegar (this takes about 3 days, so its best to set up on a Friday). Once the shell is gone, students carefully transfer their naked eggs to hypotonic distilled water, and a hypertonic solution like corn syrup or molasses. Students compare the mass of the egg before and after soaking and figure out which way the water moved while attempting to reach equilibrium.

PROS: Students love this lab! Also, eggs are round like red blood cells, so they can visualize what happens to cells in different hypertonic or hypotonic solutions.
CONS: It can get expensive to purchase enough eggs for all of your classes. Also, expect some to break along the way, so soak some extras just in case. Overall, it feels a little wasteful of perfectly good eggs. Maybe do this one as a demonstration, and then choose one of the options below for students to do in groups.
2. PURPLE ONION SKIN
Of all the osmosis labs, this one might be my favorite because I’m partial to getting out the microscopes. In this lab, students get a small piece of onion skin and make a wet mount slide using fresh water. They will see nice rectangular purple onion cells. Next, they will swap out the fresh water for salt water, and watch the cytoplasm in each cell shrivel up. If your students already know how to use microscopes, this one is a hit! Here is a full blog post with more details and pictures.
Note: I used to use elodea for this lab, but it became difficult to find at pet stores since it is invasive in many states. Purple onion is easy and cheaper! You can also find a lab write-up here .

PROS: Inexpensive, and fool proof. It is impossible to mess up! Unlike other options, this lab uses real plant cells. Also, it is the only version that doesn’t require soaking things overnight so you can get it done in one class period.
CONS: Your classroom will smell like onion for a day.
3. DIALYSIS TUBING
This version is a little fancier, and is a great option if you have honors or AP students. In this lab, students will get dialysis tubing and fill them with varying concentrations of sugar water solution. They will measure the initial mass of the tubes, and then soak the dialysis tubing overnight in distilled water. The following day they will measure the new mass, and see how water moved across the dialysis tubing membrane. You can find a free version of this lab from Amy Brown on TpT.
PROS: Students collect quantitative data, and it feels more “scientific” than other options.
CONS: Dialysis tubing is expensive, and if students don’t tie the string tight enough they can leak.

4. GUMMY BEARS
I am the first to admit gummy bears are not my favorite option, but I know many teachers who love doing it this way. In this version, students soak gummy bears in tap water, distilled water, and salt water overnight. They measure the change in the size of the gummy bear using rulers.
PROS: A large bag of gummy bears is only a few dollars, and students always love working with candy.

CONS: I don’t love this lab because depending on the brand you buy, the gummy bears can begin to dissolve and fall apart. Also, since they are an irregular shape it is difficult to calculate the change in volume.
5. WATER BEADS
An alternative to gummy bears is water beads, or Orbeez. You set it up the same way by soaking them in fresh water and salt water. If your kid already has some at home, use them instead of gummy bears. I like them better for a few reasons:
PROS: Water beads won’t fall apart like gummy bears, even after soaking multiple days. Since they are round, you can have students measure the diameter, and calculate the volume of the sphere. Also, you can dry them out afterward, place them in a Tupperware, and re-use them the following year.

CONS: Orbeez are smaller than gummy bears, so if your students struggle taking small, accurate measurements that might be a point of struggle. (Note: Beware of cheaper off-brands you can find on Amazon, because they WILL fall part unlike Orbeez).
6. BABY CARROTS
Last but not least are baby carrots! In this lab, each lab group gets 2 baby carrots. Just like other labs, they measure the mass before and after soaking them in fresh and salt water. Students will also notice that the baby carrot soaked in salt water becomes flimsy and bendable overnight. If you have already covered organelles, this can lead to a discussion about vacuoles and how plant cells become limp and flimsy when they lose water. You can find a lab write-up on my website !

PROS: Inexpensive materials, and uses real plant cells.
CONS: You won’t see the size of the carrot change, only the flexible vs stiff texture.
Alright, which is your favorite? Choose one (or two) and have a blast!

- Read more about: Cells

Hi, I'm Becca!
Search the site, browse by category.
- Back to School
- Biochemistry
- Body Systems
- Classification
- Classroom Decor
- Classroom Management
- Distance Learning
- End of the School Year
- Experiments
- Field Trips
- For NEW Teachers
- Formative Assessment
- Media in the Classroom
- Microscopes
- Photosynthesis & Respiration
- Plate Tectonics
- Sustainability
- Teacher Tips
- Weather and Climate
Get Freebies!
You might also like....

Teaching DNA Structure and Replication just got easier

10 Atmosphere Lessons for High School

Read Aloud Closure Assignments

Want a fun way to practice science vocabulary? Try out seek and finds!

Privacy Overview
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School
What is Osmosis?
September 21, 2018 By Emma Vanstone 1 Comment
I still remember learning about osmosis at school many years ago. I don’t know why that particular memory has stayed with me so strongly, maybe because it was hard to understand. Whatever the reason, osmosis is a term I’ve never forgotten the meaning of.
Definition of Osmosis
Osmosis is the net movement of water molecules across a partially permeable membrane from a region of higher water concentration to a region of lower water concentration.
Osmosis is the net movement of water molecules across a partially permeable membrane from an area of lower solute concentration to an area of higher solute concentration.
The important thing to remember is that osmosis is the movement of WATER MOLECULES ( or other solvent ), not the particles dissolved in the water. For example if you split a beaker of water into two halves with a semi permeable membrane and added salt to one side, water would move from the side of the beaker with no salt into the salty side.
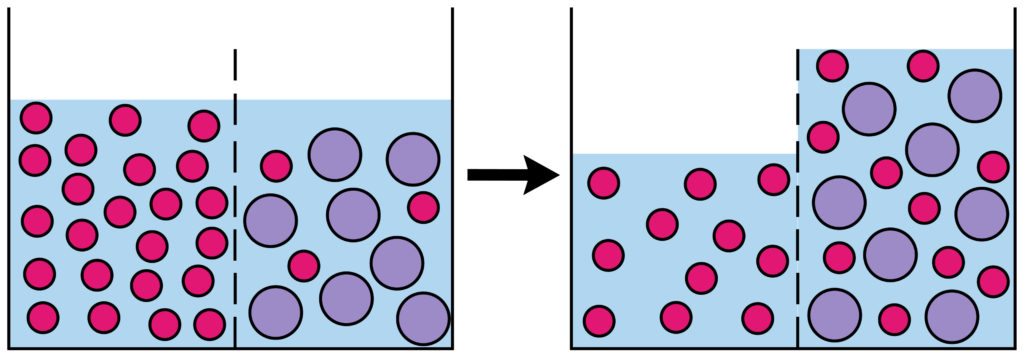
What is a partially permeable membrane?
A partially permeable membrane has very small holes in it. Tiny water molecules can fit through, but not bigger molecules like sugars.
Osmosis Example
Try soaking a raisin in water. What happens? It should swell up a little. This is because the water moves from where it is in high concentration ( the water ) into the raisins which have a low water concentration. Water keeps moving by osmosis until equilibrium is reached, this is when the concentration of both solutions is the same.
Another fun way to illustrate osmosis is with eggs as they have a handy semi-permeable membrane.
Easy Osmosis Experiment
You’ll need.
Two glasses or jars
Remove the shell from two eggs
Place two eggs in a container of vinegar for about 24 hours. The eggs should be completely submerged.
After about 24 hours, remove the eggs and gently rub the shell under cold running water. You should be able to remove most of the shell.
If it won’t all rub off, put the eggs in fresh vinegar for another few hours.

Shrink an egg
To shrink the egg you need to put in in a concentrated solution so water molecules will move from the egg into the solution..
Stir about three tablespoons of sugar into a glass of water and stir until all the sugar has dissolved.
Place one egg in this solution.
Grow an egg
Place the second egg in a glass of plain water.
Leave the eggs for about 24 hours. Can you predict what will happen?
Note how the egg in the water sinks to the bottom of the glass while the one in the sugar solution floats. This is because the sugar solution is more dense than the water.

Our egg in the water expanded while the egg in the sugar solution shrank. I used dark sugar, which is why the solution looks brown/red, but any sugar will work.

Prick the egg from the water with a fine needle and watch a jet of water shoot out!

How do you think you could rehydrate your shrunken egg?


Why does the egg grow and shrink?
Our concentrated solution was the sugar solution. The dissolved sugar molecules cannot pass through the semi-permeable membrane of the egg, but the smaller water molecules can. Water molecules move from where they are in higher concentration ( inside the egg ) to where they are in lower concentration ( the sugar solution ) until equilibrium is reached. Therefore water molecules move from inside the egg to the sugar solution. This makes the egg shrink as the net movement of water is out of the egg.
To rehydrate the egg, place it in plain water. In this instance the concentration of water molecules is higher in the water than inside the egg so the net movement of water molecules is from the water into the egg!
When we pricked the egg soaked in water, water shot out. This is because the egg had absorbed so much extra water that the pressure inside increased.
Why does egg shell dissolve in vinegar?
The egg shell dissolves in the vinegar as the acetic acid reacts with the calcium carbonate of the shell. Carbon dioxide is given off during this reaction, which is the bubbles of gas you see.
More osmosis experiments
Weigh the eggs at each stage to monitor the loss and gain of water.
Add food colouring to the water and watch the eggs absorb the coloured liquid.
What do you think would happen if you left an egg in a glass of golden syrup?

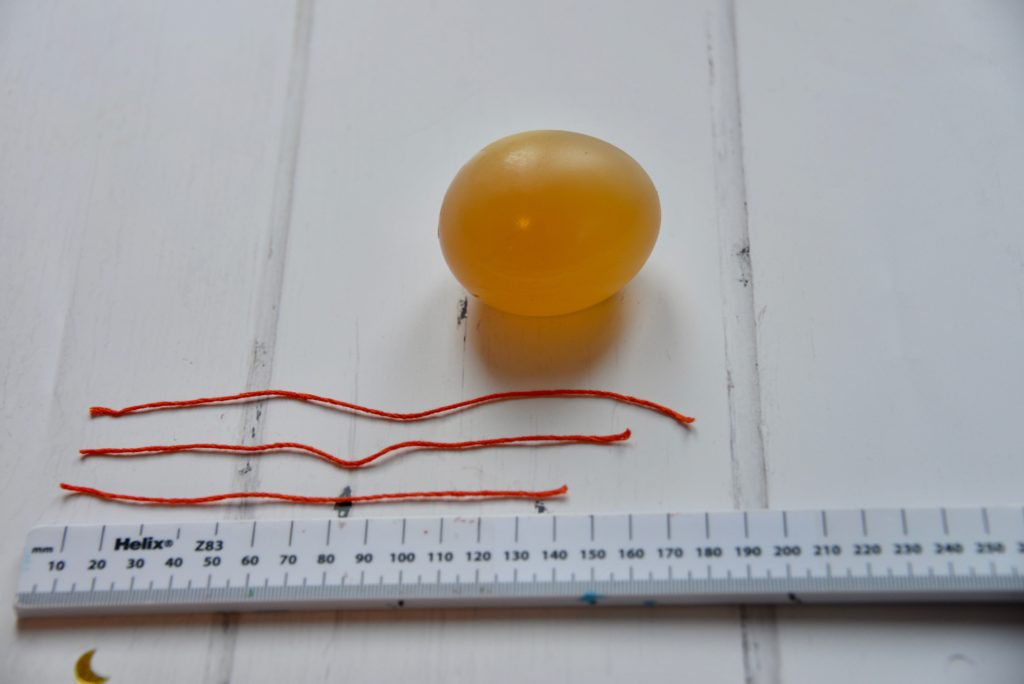
Try measuring the egg at each stage of the investigation.
We used thread to measure the diameter of the egg at its widest point after the shell was first removed, after soaking in vinegar and after soaking in golden syrup.
The longest thread is from when the egg was soaked in water. This is because the concentration of water inside the egg was lower than outside the egg, so water moved into the egg.
The shortest thread is from the egg soaked in golden syrup. Water moved by osmosis out of the egg into the golden syrup because the concentration of water inside the egg was higher than outside.
Don’t forget to wash your hands after handling raw eggs
More egg experiments for kids
Learn about tooth decay with eggs . Did you know an egg shell is similar to the outer coating on our teeth?

Find out how to transform egg white into meringue and make a tasty dessert at the same time.
Finally, did you know you can make an egg bounce ?

Last Updated on September 19, 2024 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
March 18, 2019 at 3:33 pm
I would like to receive your newsletter, please?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

Search My Blog
Very simple diffusion and osmosis experiment.

5 comments:
Good blog. Very easy to understand the point of each piece of text.

How do you make your starch solution and glucose solution?

Since no quantitative data is being collected, there is no need to make solutions of a specific concentration. I put some corn starch in a beaker of water and boil it until it clears up a bit. I put several teaspoons of Karo syrup in a beaker of water and stir until throughly mixed.
where can i find these dialysis tubing??
We order them with our lab supplies. You can also use a plastic sandwich bag.
Osmosis Science Activities For Kids

The concept of osmosis is taught to most grade school children at some level. Osmosis is a process wherein fluid passes through semi-permeable membranes from an area of high concentration to one of lower concentration. To demonstrate to kids how osmosis occurs in everyday objects, you can conduct simple, inexpensive experiments at home or in the classroom.
Colored Celery
In this experiment, kids will be able to watch how dye is transported from a cup up through a celery stalk, demonstrating the process of osmosis. You will need a bunch of fresh celery with its leaves intact, a clear cup and food coloring.
Place twenty drops of food coloring in the clear cup, and place a stalk of celery in the dye. After a few minutes, you will be able to see the dye being drawn up through the stalk of celery, into its leaves. This is a result of osmosis, and is how many plants are able to obtain the moisture they need to live from water that is in the ground.
Vinegar and Eggs
For this experiment, you will need a tape measure, clear container with a lid, one egg, large spoon and distilled white vinegar. First, measure and then record the circumference of the raw egg. Place the same egg in the container, and cover it with distilled vinegar. Allow your kids to write down their observations, then place the container in the refrigerator for 24 hours. Have the kids take a look at the egg after this time and write down what they observe, Return the egg to the refrigerator for 24 more hours.
Once the second 24 hours have passes, remove the container from the refrigerator, and carefully take the egg out of the container with a large spoon. Re-measure the circumference of the egg, and discuss the cause of the changes the kids witness.
What happened to the egg is the vinegar reacted with the calcium carbonate in the egg's shell to create bubbles. Over the 48 hours, the eggshell was dissolved by the reaction with the vinegar, although the membrane of the egg remained intact. The semipermeable membrane of the egg allowed vinegar to pass through it via osmosis. As a result, the egg itself got larger. This is a demonstration of osmosis.
Mushed Potato
To experiment with osmosis using potatoes, you will need two shallow dishes, a potato, knife, water and salt.
Fill both of the dishes with an inch of water. Add two tablespoons of salt to only one dish, while leaving the other plain. (Be sure to label which dish is plain and which has salt added to it.) Slice the potato lengthwise, so that you end up with several pieces that are flat on both sides. Place a few pieces of potato into the plain water, and an equal number of pieces into the salty water.
Allow the potatoes to sit for 20 minutes, then come back and allow the children to make their observations, and explain to them what happened.
The potatoes that were in the salt water now appear mushy because of osmosis. Due to the higher concentration of salt in the water surrounding the potatoes, the water moved from the potatoes and into the surrounding water to balance it out. This left the potatoes in the salt water mushy, while the ones in the plain water have no change to their appearance.
- Kids Craft Zone: Watch Osmosis Take Place
- Science Spot: Egg-cellent Ideas for Osmosis and Diffusion
Cite This Article
Gaines, Melinda. "Osmosis Science Activities For Kids" sciencing.com , https://www.sciencing.com/osmosis-science-activities-kids-6364184/. 22 November 2019.
Gaines, Melinda. (2019, November 22). Osmosis Science Activities For Kids. sciencing.com . Retrieved from https://www.sciencing.com/osmosis-science-activities-kids-6364184/
Gaines, Melinda. Osmosis Science Activities For Kids last modified August 30, 2022. https://www.sciencing.com/osmosis-science-activities-kids-6364184/
Recommended

- Plant Biology
- Human Biology
- Biology Cells Osmosis Experiment
Osmosis Experiment: Dissolving Egg Shells With Vinegar
How does osmosis keep you healthy.
Right now, as you read this, there are millions of things happening throughout your body. The food you ate just a bit ago is making its way through a watery slurry inside your stomach and small intestines. Your kidneys are working hard to excrete waste and extra water. The lacrimal glands near your eyes are secreting tears, which allow your eyelids to close without damaging your eyeballs. What’s one thing that all of these processes have in common? They all rely on osmosis: the diffusion of water from one place to another.
Osmosis factors heavily in each of these processes and is an important force for keeping every single cell in your body healthy. Osmosis is hard to see without a microscope. But if we create our very own model of a cell, using a shell-less chicken egg, we can see what happens when we manipulate the osmotic balance in the “cell”!

- 3 glasses (large enough to fit the egg plus liquid)
- 3 butter knives
- White vinegar (about 3 cups)
- Distilled water (about 2 cups)
- Light corn syrup (about 1 ¼ cups)
- Slotted spoon
- Measuring cup (1 cup)
- Measuring spoons (1 tablespoon and ½ tablespoon)
- Sticky notes and marker
- Scale (optional)
Note : It’s okay to touch the eggs, but remember to wash your hands afterwards to avoid any nasty surprises!
1. Place one egg in each glass. Pour in enough vinegar to cover each egg. Bubbles will start to form around the egg, and it’ll float up. To keep it submerged, put a butter knife in the glass to hold it down.
2. Put the three glasses in the refrigerator and allow to sit for 24 hours.
3. Gently holding the egg in the glass, pour out the old vinegar. Replace with fresh vinegar, and let sit in the refrigerator for another 24 hours. Repeat this process until the shells are fully dissolved and only the membrane remains. This should take about 2-3 days.
4. Gently remove the eggs using the slotted spoon and rinse with tap water in the sink. Rinse out the empty glasses as well.
5. Gently put the shell-less eggs aside for a moment on a plate.
6. Prepare three different sugar-water solutions as follows, labeling with sticky notes:
Glass 1: Label “hypertonic”. Pour in one cup of corn syrup.
Glass 2: Label “isotonic”. Add 1 ½ tablespoons corn syrup to the one cup measuring cup, and fill the remainder with distilled water. Pour into glass (make sure you get all the corn syrup out!) and stir to dissolve.
Glass 3: Label “hypotonic”. Pour in one cup of distilled water. Gently put one shell-less egg in each of the glasses, and let sit in the refrigerator for another 24 hours.
7. Remove the glasses from the refrigerator, and gently put the eggs on a plate. If you weighed the eggs before putting them in each solution, weigh them again. What happened to each of the eggs?

How does osmosis work?
Osmosis is the scientific term that describes how water flows to different places depending on certain conditions. In this case, water moves around to different areas based on a concentration gradient , i.e. solutions which have different concentrations of dissolved particles ( solutes ) in them. Water always flows to the area with the most dissolved solutes, so that in the end both solutions have an equal concentration of solutes. Think about if you added a drop of food dye to a cup of water – even if you didn’t stir it, it would eventually dissolve on its own into the water.
In biological systems, the different solutions are usually separated by a semipermeable membrane , like cell membranes or kidney tubules . These act sort of like a net that keeps solutes trapped, but they still allow water to pass through freely. In this way, cells can keep all of their “guts” contained but still exchange water.
Now, think about the inside of an egg. There’s a lot of water inside of the egg, but a lot of other things (i.e. solutes) too, like protein and fat. When you placed the egg in the three solutions, how do you think the concentration of solutes differed between the inside of the egg and outside of the egg? The egg membrane acts as a semipermeable membrane and keeps all of the dissolved solutes separated but allows the water to pass through.
How did osmosis make the eggs change size (or not)?
If the steps above work out properly, the results should be as follows.
In the case of the hypertonic solution, there were more solutes in the corn syrup than there were in the egg. So, water flowed out of the egg and into the corn syrup, and as a result the egg shriveled up.
In the case of the isotonic solution, there was roughly an equal amount of solutes in the corn syrup/water solution than there was in the egg, so there was no net movement in or out of the egg. It stayed the same size.
In the case of the hypotonic solution, there were more solutes in the egg than in the pure water. So, water flowed into the egg, and as a result, it grew in size.

Osmosis and You
Every cell in your body needs the right amount of water inside of it to keep its shape, produce energy, get rid of wastes, and other functions that keep you healthy.
This is why medicines that are injected into patients need to be carefully designed so that the solution has the same concentration of solutes as their cells (i.e. isotonic). If you were sick and became dehydrated, for example, you would get a 0.90% saline IV drip. If it were too far off from this mark it wouldn’t be isotonic anymore, and your blood cells might shrivel up or even explode , depending on the concentration of dissolved solutes in the water.
Osmosis works just the same way in your cells as it does in our egg “cell” model. Thankfully, though, the semipermeable membrane of the egg is much stronger, so you don’t have to worry about the egg exploding as well!
Related Topics
Choose one of the following categories to see related pages:.
- Experiments
Share this Page
Lindsay graduated with a master’s degree in wildlife biology and conservation from the University of Alaska Fairbanks. She also spent her time in Alaska racing sled dogs, and studying caribou and how well they are able to digest nutrients from their foods. Now, she enjoys sampling fine craft beers in Fort Collins, Colorado, knitting, and helping to inspire people to learn more about wildlife, nature, and science in general.
- Basic Types of Cells
- Cell Organelles
- Connective Tissue Cells
- Epithelial Cells
- Introduction to Cells
- Muscle Cells
- Nerve Cells
- Osmosis Experiment
- Structure of the Cell Nucleus
- Structures of the Cell Cytoplasm
Science Newsletter:
Full list of our videos.

Teaching Biology?

How to Make Science Films

Read our Wildlife Guide

New From Untamed Science


IMAGES
VIDEO
COMMENTS
Osmosis is the diffusion of water across a semipermeable membrane. Use gummy bears to demonstrate osmosis with this simple science experiment.
There are quite a few ways to easily see osmosis in action! Here’s a round-up of six labs and the pros and cons of each: 1. EGG OSMOSIS. The “naked egg” lab is very popular in biology. Students really get a kick out of seeing the eggshell dissolve!
Osmosis made easy. Fun and simple osmosis experiments for kids. Perfect for Key Stage 3 Science or for a great science fair project.
Water uptake in plants is quite complex and involves a process called osmosis. Osmosis makes the water from the soil move into the roots of the plant. But what drives the water from the soil into the plant cells? In this activity, you will do an experiment with potatoes to find out!
Learn about osmosis with this simple science experiment using potato slices. Discover how water and salt affect freshness and texture in this easy-to-follow activity.
Since the gummy bear water was removed when it was created, when you place a gummy bear in water the water will move into the bear by osmosis. But in which liquid will the gummy bear grow the most? Find out as we use the scientific method in this gummy bear science experiment!
Just this week, our biology students completed an activity that is so very simple, but it really illustrates the concept of semipermeable membranes. Students are given 2 pieces of dialysis tubing. One is filled with a starch solution and the other is filled with a glucose solution.
To demonstrate to kids how osmosis occurs in everyday objects, you can conduct simple, inexpensive experiments at home or in the classroom. In this experiment, kids will be able to watch how dye is transported from a cup up through a celery stalk, demonstrating the process of osmosis.
Osmosis helps regulate the amount of water in an organism's cells to help it keep cell shape and function for the health of the whole body.
The below mentioned article includes a list of four simple experiments on osmosis. 1. Experiment to demonstrate the osmosis by using sheet of cellophane or goat bladder: Requirements: Beaker, thistle funnel, goat bladder or sheet of cellophane, thread, water and sugar solution.