Suggestions or feedback?

MIT News | Massachusetts Institute of Technology
- Machine learning
- Sustainability
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
New autism research projects represent a broad range of approaches to achieving a shared goal
Press contact :.

Previous image Next image
From studies of the connections between neurons to interactions between the nervous and immune systems to the complex ways in which people understand not just language, but also the unspoken nuances of conversation, new research projects at MIT supported by the Simons Center for the Social Brain are bringing a rich diversity of perspectives to advancing the field’s understanding of autism.
As six speakers lined up to describe their projects at a Simons Center symposium Nov. 15, MIT School of Science dean Nergis Mavalvala articulated what they were all striving for: “Ultimately, we want to seek understanding — not just the type that tells us how physiological differences in the inner workings of the brain produce differences in behavior and cognition, but also the kind of understanding that improves inclusion and quality of life for people living with autism spectrum disorders.”
Simons Center director Mriganka Sur , Newton Professor of Neuroscience in The Picower Institute for Learning and Memory and Department of Brain and Cognitive Sciences (BCS), said that even though the field still lacks mechanism-based treatments or reliable biomarkers for autism spectrum disorders, he is optimistic about the discoveries and new research MIT has been able to contribute. MIT research has led to five clinical trials so far, and he praised the potential for future discovery, for instance in the projects showcased at the symposium.
“We are, I believe, at a frontier — at a moment where a lot of basic science is coming together with the vision that we could use that science for the betterment of people,” Sur said.
The Simons Center funds that basic science research in two main ways that each encourage collaboration, Sur said: large-scale projects led by faculty members across several labs, and fellowships for postdocs who are mentored by two faculty members, thereby bringing together two labs. The symposium featured talks and panel discussions by faculty and fellows leading new research.

Previous item Next item
In a different vein, Associate Professor Ev Fedorenko of The McGovern Institute for Brain Research and BCS is leading a seven-lab collaboration aimed at understanding the cognitive and neural infrastructure that enables people to engage in conversation, which involves not only the language spoken but also facial expressions, tone of voice, and social context. Critical to this effort, she said, is going beyond previous work that studied each related brain area in isolation to understand the capability as a unified whole. A key insight, she said, is that they are all nearby each other in the lateral temporal cortex.
“Going beyond these individual components we can start asking big questions like, what are the broad organizing principles of this part of the brain?,” Fedorenko said. “Why does it have this particular arrangement of areas, and how do these work together to exchange information to create the unified percept of another individual we’re interacting with?”
While Choi and Fedorenko are looking at factors that account for differences in social behavior in autism, Picower Professor Earl K. Miller of The Picower Institute and BCS is leading a project that focuses on another phenomenon: the feeling of sensory overload that many autistic people experience. Research in Miller’s lab has shown that the brain’s ability to make predictions about sensory stimuli, which is critical to filtering out mundane signals so attention can be focused on new ones, depends on a cortex-wide coordination of the activity of millions of neurons implemented by high frequency “gamma” brain waves and lower-frequency “beta” waves. Working with animal models and human volunteers at Boston Children’s Hospital (BCH), Miller said his team is testing the idea that there may be a key difference in these brain wave dynamics in the autistic brain that could be addressed with closed-loop brain wave stimulation technology.
Simons postdoc Lukas Vogelsang , who is based in BCS Professor Pawan Sinha’s lab, is looking at potential differences in prediction between autistic and non-autistic individuals in a different way: through experiments with volunteers that aim to tease out how these differences are manifest in behavior. For instance, he’s finding that in at least one prediction task that requires participants to discern the probability of an event from provided cues, autistic people exhibit lower performance levels and undervalue the predictive significance of the cues, while non-autistic people slightly overvalue it. Vogelsang is co-advised by BCH researcher and Harvard Medical School Professor Charles Nelson.

Fundamentally, the broad-scale behaviors that emerge from coordinated brain-wide neural activity begins with the molecular details of how neurons connect with each other at circuit junctions called synapses. In her research based in The Picower Institute lab of Menicon Professor Troy Littleton, Simons postdoc Chhavi Sood is using the genetically manipulable model of the fruit fly to investigate how mutations in the autism-associated protein FMRP may alter the expression of molecular gates regulating ion exchange at the synapse , which would in turn affect how frequently and strongly a pre-synaptic neuron excites a post-synaptic one. The differences she is investigating may be a molecular mechanism underlying neural hyperexcitability in fragile X syndrome, a profound autism spectrum disorder.
In her talk, Simons postdoc Lace Riggs , based in The McGovern Institute lab of Poitras Professor of Neuroscience Guoping Feng, emphasized how many autism-associated mutations in synaptic proteins promote pathological anxiety. She described her research that is aimed at discerning where in the brain’s neural circuitry that vulnerability might lie. In her ongoing work, Riggs is zeroing in on a novel thalamocortical circuit between the anteromedial nucleus of the thalamus and the cingulate cortex, which she found drives anxiogenic states. Riggs is co-supervised by Professor Fan Wang.
After the wide-ranging talks, supplemented by further discussion at the panels, the last word came via video conference from Kelsey Martin, executive vice president of the Simons Foundation Autism Research Initiative . Martin emphasized that fundamental research, like that done at the Simons Center, is the key to developing future therapies and other means of supporting members of the autism community.
“We believe so strongly that understanding the basic mechanisms of autism is critical to being able to develop translational and clinical approaches that are going to impact the lives of autistic individuals and their families,” she said.
From studies of synapses to circuits to behavior, MIT researchers and their collaborators are striving for exactly that impact.
Share this news article on:
Related links.
- Simons Center for the Social Brain
- McGovern Institute for Brain Research
- Department of Brain and Cognitive Sciences
Related Topics
- Special events and guest speakers
- Brain and cognitive sciences
- Neuroscience
- Picower Institute
- McGovern Institute
Related Articles

“UnrulyArt” creates joy and engagement, regardless of ability

Understanding why autism symptoms sometimes improve amid fever
Simons Center’s collaborative approach propels autism research, at MIT and beyond
More mit news.

Ecologists find computer vision models’ blind spots in retrieving wildlife images
Read full story →
Tiny, wireless antennas use light to monitor cellular communication

MIT-Kalaniyot launches programs for visiting Israeli scholars

Global MIT At-Risk Fellows Program expands to invite Palestinian scholars

Startup’s autonomous drones precisely track warehouse inventories

MIT affiliates receive 2025 IEEE honors
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
New autism research projects represent a broad range of approaches to achieving a shared goal
From studies of the connections between neurons to interactions between the nervous and immune systems to the complex ways in which people understand not just language, but also the unspoken nuances of conversation, new research projects at MIT supported by the Simons Center for the Social Brain are bringing a rich diversity of perspectives to advancing the field’s understanding of autism.
As six speakers lined up to describe their projects at a Simons Center symposium Nov. 15, MIT School of Science dean Nergis Mavalvala articulated what they were all striving for: “Ultimately, we want to seek understanding — not just the type that tells us how physiological differences in the inner workings of the brain produce differences in behavior and cognition, but also the kind of understanding that improves inclusion and quality of life for people living with autism spectrum disorders.”
Simons Center director Mriganka Sur , Newton Professor of Neuroscience in The Picower Institute for Learning and Memory and Department of Brain and Cognitive Sciences (BCS), said that even though the field still lacks mechanism-based treatments or reliable biomarkers for autism spectrum disorders, he is optimistic about the discoveries and new research MIT has been able to contribute. MIT research has led to five clinical trials so far, and he praised the potential for future discovery, for instance in the projects showcased at the symposium.
“We are, I believe, at a frontier — at a moment where a lot of basic science is coming together with the vision that we could use that science for the betterment of people,” Sur said.
The Simons Center funds that basic science research in two main ways that each encourage collaboration, Sur said: large-scale projects led by faculty members across several labs, and fellowships for postdocs who are mentored by two faculty members, thereby bringing together two labs. The symposium featured talks and panel discussions by faculty and fellows leading new research.
In a different vein, Associate Professor Ev Fedorenko of The McGovern Institute for Brain Research and BCS is leading a seven-lab collaboration aimed at understanding the cognitive and neural infrastructure that enables people to engage in conversation, which involves not only the language spoken but also facial expressions, tone of voice, and social context. Critical to this effort, she said, is going beyond previous work that studied each related brain area in isolation to understand the capability as a unified whole. A key insight, she said, is that they are all nearby each other in the lateral temporal cortex.
“Going beyond these individual components we can start asking big questions like, what are the broad organizing principles of this part of the brain?,” Fedorenko said. “Why does it have this particular arrangement of areas, and how do these work together to exchange information to create the unified percept of another individual we’re interacting with?”
While Choi and Fedorenko are looking at factors that account for differences in social behavior in autism, Picower Professor Earl K. Miller of The Picower Institute and BCS is leading a project that focuses on another phenomenon: the feeling of sensory overload that many autistic people experience. Research in Miller’s lab has shown that the brain’s ability to make predictions about sensory stimuli, which is critical to filtering out mundane signals so attention can be focused on new ones, depends on a cortex-wide coordination of the activity of millions of neurons implemented by high frequency “gamma” brain waves and lower-frequency “beta” waves. Working with animal models and human volunteers at Boston Children’s Hospital (BCH), Miller said his team is testing the idea that there may be a key difference in these brain wave dynamics in the autistic brain that could be addressed with closed-loop brain wave stimulation technology.
Simons postdoc Lukas Vogelsang , who is based in BCS Professor Pawan Sinha’s lab, is looking at potential differences in prediction between autistic and non-autistic individuals in a different way: through experiments with volunteers that aim to tease out how these differences are manifest in behavior. For instance, he’s finding that in at least one prediction task that requires participants to discern the probability of an event from provided cues, autistic people exhibit lower performance levels and undervalue the predictive significance of the cues, while non-autistic people slightly overvalue it. Vogelsang is co-advised by BCH researcher and Harvard Medical School Professor Charles Nelson.
Fundamentally, the broad-scale behaviors that emerge from coordinated brain-wide neural activity begins with the molecular details of how neurons connect with each other at circuit junctions called synapses. In her research based in The Picower Institute lab of Menicon Professor Troy Littleton, Simons postdoc Chhavi Sood is using the genetically manipulable model of the fruit fly to investigate how mutations in the autism-associated protein FMRP may alter the expression of molecular gates regulating ion exchange at the synapse , which would in turn affect how frequently and strongly a pre-synaptic neuron excites a post-synaptic one. The differences she is investigating may be a molecular mechanism underlying neural hyperexcitability in fragile X syndrome, a profound autism spectrum disorder.
In her talk, Simons postdoc Lace Riggs , based in The McGovern Institute lab of Poitras Professor of Neuroscience Guoping Feng, emphasized how many autism-associated mutations in synaptic proteins promote pathological anxiety. She described her research that is aimed at discerning where in the brain’s neural circuitry that vulnerability might lie. In her ongoing work, Riggs is zeroing in on a novel thalamocortical circuit between the anteromedial nucleus of the thalamus and the cingulate cortex, which she found drives anxiogenic states. Riggs is co-supervised by Professor Fan Wang.
After the wide-ranging talks, supplemented by further discussion at the panels, the last word came via video conference from Kelsey Martin, executive vice president of the Simons Foundation Autism Research Initiative . Martin emphasized that fundamental research, like that done at the Simons Center, is the key to developing future therapies and other means of supporting members of the autism community.
“We believe so strongly that understanding the basic mechanisms of autism is critical to being able to develop translational and clinical approaches that are going to impact the lives of autistic individuals and their families,” she said.
From studies of synapses to circuits to behavior, MIT researchers and their collaborators are striving for exactly that impact.
Autism Research in 2022
Written by staff and the SAB of the Autism Science Foundation

COVID Recovery Was Slow, But Scientific Progress Continues
After two grueling pandemic years, 2022 saw a return to quasi-normalcy in both the world at large and in the world of autism research. Although the pandemic was incredibly challenging for autism families and researchers, the pivot to telehealth led to advances in online autism diagnoses, mental health supports, and interventions that will likely benefit autistic people across the globe for years to come.
Autism scientists working in diverse areas of research made important strides this year and continued to gain valuable insights into every facet of autism. They also identified more effective ways to support people on the spectrum. Researchers developed a better understanding of the unique needs and priorities of specific groups of autistic people , better-defined links between biological mechanisms and behavior , and disparities in autism diagnosis and treatment.
This work was only possible because of families like yours: You actively participated in important research studies. You agreed to have your data shared with others. You donated. You advocated. Your U.S. tax dollars supported $100 million of NIH grants funded in 2022 .

Autism science simply cannot progress without your continued partnership. Earlier this year, ASF launched a “ Participate in Research ” directory to match families with research studies that meet your needs and interests. Many of these studies offer compensation, and can also provide valuable information and resources to aid your family member. The goal is to use the information gleaned from research to improve the real lives of real people, both now and in the future.
Here’s a little bit of what 2022 taught us.
Early identification leads to earlier diagnosis, but diagnosis happens at all ages
- Developmental milestones are skills that most children reach at a certain age and are used by healthcare providers to track progress. This year, the CDC updated these milestones to track what 75% of children can do by certain ages, rather than 50%, causing some pushback. In addition, the CDC added new time points as well as markers that might predict an autism diagnosis. 1
- In autism, reaching developmental milestones can be delayed from months to years. Delays are often more severe and variable in those with co-occurring intellectual disability and a rare genetic variant. New research reinforced the need to focus on milestones and the importance of early intervention.: If you notice your infant is struggling with new skills, tell your healthcare provider. 2
- Language skills in infants are an important predictor of an ASD diagnosis. Recent work from the ASF-supported Baby Siblings Research Consortium (BSRC) showed that maternal education levels and early gestures are important predictors of these language skills, suggesting markers for intervention. 3
- Researchers have suggested that early behaviors that are predictive of a later diagnosis may be part of a larger “developmental cascade,” where, for example, the trajectory from laying to sitting to language may be disrupted. These are intertwined behavioral and neurobiological networks that affect how a person with autism functions. 4
- There are now multiple biomarkers under investigation. Some are better than others at not just autism diagnosis, but the response to intervention. 5 In the future, they can be used to promote earlier diagnosis and more objective measures of the effectiveness of interventions.
Key takeaways: Parents and clinicians should monitor developmental milestones early in life. Early signs are not a substitute for a diagnosis, but some supports and interventions can be provided that allow for an improvement of trajectories across the lifespan.
Parent-mediated interventions and training – they work.
- A review of 30 studies showed promising results from parent-mediated interventions, but improvements in studies are still needed. 6
- Parent-mediated interventions can be used for teaching everything from core autism symptoms to self-care like tooth brushing. 7
- Autism interventions can and should be customized to culture and race. 8,9
- Some parent-mediated interventions have been tested successfully in a hybrid format, leading the way for others to investigate their effect on parent and child outcomes. 10
- While some have suggested parents only recognize the weaknesses in their children, recent research strongly notes that parents know their child’s strengths and use those strengths to help support their family. Educators also note these strengths in the classroom. 11,12
- Siblings play an important role in the outcome of autistic individuals, while they also experience unique challenges themselves. 13,14
Key takeaways: Parents and caregivers often feel helpless when they are concerned about their child’s development and are facing long waiting lists for services. New research shows that providing support is beneficial for both the parents and the child outcome, and elevates strengths while mitigating support challenges. Further research should continue to explore the role of sibling relationships and support.

The brain has a distinct “signature” and sensory issues are on the front line
- One type of immune cell of the brain called the microglia has been known to affect cell communication, shape, and number. Researchers have now determined when and where these cells are expressed during development, laying the foundation for research into a critical brain cell type. 17
- The greatest differences in gene expression in the brain are in sensory areas like the visual cortex. 15 This may explain the almost universal problems in sensory processing that autistic individuals experience, and why sensory problems are so common in ASD. 18
- The visual area, specifically the occipital cortex, was also enlarged at young ages, more so in kids who have siblings with a diagnosis, demonstrating that genetic heritability plays a role in brain activity involved in sensory processing in families. 19
- A new marker of sensory processing was detected: differences in the activity of a neurotransmitter called GABA. GABA commonly slows down the activity of brain cells, which is important when they are too active, indicating this neurotransmitter is critical for sensory processing. Changing the activity of GABA neurons can alleviate sensory problems in autistic individuals. 20
- In addition, changes in the thickness of different cortical regions may influence sensory responses, depending on whether there is overstimulation or understimulation. 21
- Another brain region called the amygdala may relate to anxiety in autistic people. Certain areas of the amygdala are different in size, 22 and can explain variability in anxiety. 23 There is also disruption in connectivity from the amygdala to outside regions, 24 which may also explain how anxiety interacts with autism features.
- Rather than examining one autism feature at a time, it seems that ability to make gains or show potential for change over time is correlated to differences in brain structure. Markers of change over time are also linked to genes associated with ASD. 25 Targets of intervention based on biological markers may need to focus on sensitivity to change rather than a specific number on an instrument per se.
- The use of biological tools has increased this year. These tools include induced pluripotent stem cells (IPSCs) and organoids that are based on cells from individuals with different forms of ASD. Studies have looked at different types of autism (idiopathic and genetically-based) and identified creation of new brain cells as a common biological mechanism. 26 New studies also used novel tools to improve the validity of these cell-based systems. 27
- Animal models can be used to identify mechanisms by which genes and environmental factors exert their influence over behavior. Right now, there are hundreds of animal models of ASD, but not all of them are used appropriately to understand ASD. The ability of the model to recapitulate both the biology and behavior involved in ASD is essential. 28
Key takeaways: While different brain regions are specialized in their function, they interconnect and turn on and off in synchrony. Researchers need better models of human neurobiology, including better animal models, to understand the core and associated autism features, from sensory dysfunction to GI issues. If you want to learn more about research involving the brains of people with autism, sign up for more information at Autism BrainNet .
Genetic markers start to explain phenotype.

- The presence of rare genetic variants and common variants tend to funnel people into groups defined by intellectual disability (ID) or high educational attainment. 29,30 Scientists have identified and characterized two major types of genetic variation associated with ASD. Rare genetic variants are commonly associated with lower cognitive function and profound autism, but that is not always the case. 31 Even with hundreds of thousands of samples, scientists have still not found a direct gene – outcome linkage. However, genetics are still important. Genetic findings can help identify specific needs leading to appropriate supports.
- Certain types of gene mutations can explain associations with features like psychosis, 32 as well as obesity and depression. 33
- Five new variants were identified that are not linked to intellectual or developmental disability (IDD), but are linked to other neuropsychiatric issues besides ASD. 31,34 Therefore, rare ASD or DD gene mutations usually lead to some sort of deleterious outcome.
- There is a significant overlap between ASD genes and genes associated with developmental disorders in general. Researchers suggest that autism specificity may be the result of when the gene is expressed. For example, in developmental disorders, genes are expressed in progenitor cells while in ASD they may be expressed in developing neurons. 35
- Other studies have not found any ASD-specific gene, they show linkage to neurodevelopmental problems in general, and can be grouped based on what cells are affected. 35
- There are shared pathways between ASD and other neuropsychiatric disorders. 36
- Studies have shown linkages between epilepsy, ASD and ADHD. 37
Key takeaways: Genetic markers associated with ASD are also associated with other developmental conditions like ADHD and intellectual disability, as well as comorbid conditions like obesity. Two major types of genetic markers, rare and common variations, may represent biomarkers of two different phenotypes, but there is overlap, and rare and common variants are likely mixed in most people. Genetic research is important for a better understanding of ASD and the development of individualized approaches for supports.
But genetics doesn’t tell it all.
- Parental genetics and environmental factors are intertwined on a biological level. Genes associated with depression in parents are also linked to ASD. 38
- Maternal immune infections are an established risk factor for ASD. However, the genetics of children with and without maternal immune challenges during pregnancy are different. 39
- Studies in Norway offer a unique perspective of gestational exposures by banking blood taken mid-pregnancy during usual obstetrical visits. One study has shown that certain cytokines, or markers of immune activity, are elevated during pregnancy in both boys and girls with autism, particularly in girls. It’s unclear what role these cytokines play collectively or individually, or where they came from in the first place. 40
- Where you live can affect the role of genes vs. environment, evidenced by environmental factors playing a bigger role in heritability in certain areas of Sweden and the U.K. 41
- Genetics and the environment clearly interact when it comes to the influence of an ASD diagnosis. For example, pesticide exposure exacerbated the effects of the autism CHD8 gene on rodent behavior. 42
- The role of environmental factors may depend not just on a diagnosis but on specific autism traits. 43
- Given that autism is likely part of a larger developmental disorder spectrum, regulation of toxic chemicals which are harmful to development must be expanded. 44
Key takeaways: The role of environmental factors in ASD has often been disassociated with genetics when it should be integrated into the understanding of autism’s causes, behavioral features, and interventions.
Biological sex plays a role.

- Studies replicated this year showed that females with autism have a higher burden of rare genetic mutations. In addition, research is demonstrating that females with an autism diagnosis also show a higher level of “common” variations. 29,45
- The effect of higher levels of common variation in females extends to even undiagnosed members of ASD-impacted families, demonstrating that females carrying ASD genetic variation are resilient. 45
- The two above studies implicate an important role of the female protective effect but do not explain all of the differences in diagnosis. 46
- Some scientists have wondered if biases in instruments used to inform a diagnosis play a role in the sex difference. One study used a mathematical algorithm to eliminate the difference in M:F diagnostic differences, but still, females show different behavioral profiles. This further reiterates that instruments should be used to inform, not make a diagnosis, and that autism is more than a yes or no diagnosis. 47
- Clinicians may miss an autism diagnosis in females because of camouflage. Females are also more likely to camouflage, which means they (consciously or unconsciously) pretend to fit in as a typically-developing girl. This leads to lower quality of life. 48
- Intellectual disability plays a bigger role in autism features in girls vs. boys. 49
- New genetic mutations involving the X chromosome were identified – and these mutations are more likely to occur in females. 35
- Sex differences in brain region size can be attributed to gene expression patterns. In other words, brain differences in males and females with ASD are due, in part, to underlying genetics. 50
Key takeaways: Females with ASD show different biological and behavioral profiles and are understudied in research and underserved in the community. Future research should aim to include more females to better understand their unique needs and provide targeted support.
It’s still not over, but families are in a better place than a year ago.

- Despite a rocky start at the height of the pandemic in 2020 and 2021, opportunities to receive autism diagnoses, mental health supports, and interventions via telehealth have been improved, and polished, and are not only acceptable to families and clinicians but are effective. 51-57
- Families and clinicians were happier with remote diagnosis and evaluation when the diagnosis was clear; in cases where there was some ambiguity, it caused frustration. 58,59
- While many families and individuals experienced a mental health decline during the pandemic, some exhibited resiliency under social distancing guidelines. 60 The differences could be due to the degree to which services were lost, coping styles, and pre-existing mental health attributes. 61
Key takeaways: Autism families suffered during the pandemic, but it also allowed for new approaches to be developed that may ultimately improve practice – including hybrid clinical services, holistic family support, and more comprehensive diagnostic practices.
It’s not all about the asd.
- Individuals with ASD experience higher levels of anxiety, GI issues, epilepsy, and other developmental disorders like ADHD compared to those without a diagnosis.
- While not a core autism symptom, anxiety is linked to insistence on sameness in toddlers with ASD, which indicates a similar underlying mechanism. 62
- Gastrointestinal issues plague people with autism, and there are few options for treatment. The gastrointestinal microbiome has been a target for intervention for autism symptoms, although studies are still ongoing. 63 GI issues were the focus of a major NIH-funded meeting this year .
- Suicide risk is higher in ASD. 64
- Sleep problems, while mostly studied in children, are now shown to follow kids into adolescence and adulthood. 65
- There is a high degree of overlap in the brain activity profiles between ADHD and ASD kids. Differences are mostly seen when symptom severity is accounted for. ADHD and ASD show more similarities in the brain than differences. 66
- Behavioral profiles between ADHD and ASD are also similar. 67
- Mental health concerns are present in adolescents and adults with ASD with cognitive inflexibility strongly linked to compromised mental health. 68,69 Cognitive inflexibility, which is different than cognitive ability, is how someone shifts their attention from one thing to another based on what is going on around them. This may be a focus for future mental health interventions.
- Unfortunately there are no strong individual-level predictors in childhood of mental health issues in adults, but some factors that may lead to better mental health are better living skills and higher IQ. 70
Key takeaways: Outside the core features of autism listed in the DSM5, individuals experience a wide range of associated features, ranging from psychiatric issues to medical comorbidities. For many individuals, these associated features are highly debilitating.
Biases in underserved communities are getting more attention.

- A recent analysis showed a reduction of the disparities in the age of ASD diagnosis for Black and Hispanic children over the last four years, but a difference still exists. 71
- This is likely due to provider bias, but not necessarily diagnostic instrument biases. The standard diagnostic tools are not biased toward race or sex. 72
- Lessons learned from the pandemic reiterate the need for intense community engagement, flexibility, and an understanding that a holistic approach – rather than one focused on ASD – is necessary for working with underserved communities 73,74 .
- A culturally-adapted parent training program delivered by Black providers was effective in the Black community and could be a model for future engagement efforts. 8
- Only 25% of intervention studies report the ethnic and racial makeup of their participants, 75 indicating that researchers need to do a better job of deliberately including racial and ethnic minorities, recruiting them as research leads and coordinators, and including them on boards for scientific review. 76
- Low socioeconomic status contributes to social and communication deficits in young children with ASD. 77
Key takeaways: Racial and ethnic biases are still pervasive in autism research and diagnosis, and we need a holistic approach to support families in all aspects of their lives beyond just autism symptoms. Scientists must continue to focus on the deliberate inclusion of these groups in both research and career training to better serve all individuals with autism.
On a final note, there has been a lot of debate this year about the language used to describe autism. 78-81 There is a diversity of experiences with autism and likely to be a diversity of perspectives. Families and scientists should use scientifically accurate terms to best describe the wide range of autistic people and their symptoms. 82 What that is may differ from person to person, and situation to situation, which means context and preference need to be considered as well.
1. Zubler JM, Wiggins LD, Macias MM, et al. Evidence-Informed Milestones for Developmental Surveillance Tools. Pediatrics 2022; 149 (3).
2. Kuo SS, van der Merwe C, Fu JM, et al. Developmental Variability in Autism Across 17 000 Autistic Individuals and 4000 Siblings Without an Autism Diagnosis: Comparisons by Cohort, Intellectual Disability, Genetic Etiology, and Age at Diagnosis. JAMA Pediatr 2022; 176 (9): 915-23.
3. Pecukonis M, Young GS, Brian J, et al. Early predictors of language skills at 3 years of age vary based on diagnostic outcome: A baby siblings research consortium study. Autism Res 2022; 15 (7): 1324-35.
4. Bradshaw J, Schwichtenberg AJ, Iverson JM. Capturing the complexity of autism: Applying a developmental cascades framework. Child Dev Perspect 2022; 16 (1): 18-26.
5. Webb SJ, Naples AJ, Levin AR, et al. The Autism Biomarkers Consortium for Clinical Trials: Initial Evaluation of a Battery of Candidate EEG Biomarkers. Am J Psychiatry 2022: appiajp21050485.
6. Conrad CE, Rimestad ML, Rohde JF, et al. Parent-Mediated Interventions for Children and Adolescents With Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Front Psychiatry 2021; 12 : 773604.
7. Fenning RM, Butter EM, Macklin EA, et al. Parent Training for Dental Care in Underserved Children With Autism: A Randomized Controlled Trial. Pediatrics 2022; 149 (5).
8. Kaiser K, Villalobos ME, Locke J, Iruka IU, Proctor C, Boyd B. A culturally grounded autism parent training program with Black parents. Autism 2022; 26 (3): 716-26.
9. Rivera-Figueroa K, Marfo NYA, Eigsti IM. Parental Perceptions of Autism Spectrum Disorder in Latinx and Black Sociocultural Contexts: A Systematic Review. Am J Intellect Dev Disabil 2022; 127 (1): 42-63.
10. Brian J, Solish A, Dowds E, et al. “Going Mobile”-increasing the reach of parent-mediated intervention for toddlers with ASD via group-based and virtual delivery. J Autism Dev Disord 2022; 52 (12): 5207-20.
11. Mirenda P, Zaidman-Zait A, Cost KT, et al. Educators Describe the “Best Things” About Students with Autism at School. J Autism Dev Disord 2022.
12. Wilkinson E, Vo LTV, London Z, Wilson S, Bal VH. Parent-Reported Strengths and Positive Qualities of Adolescents and Adults with Autism Spectrum Disorder and/or Intellectual Disability. J Autism Dev Disord 2022; 52 (12): 5471-82.
13. Rosen NE, Schiltz HK, Lord C. Sibling Influences on Trajectories of Maladaptive Behaviors in Autism. J Clin Med 2022; 11 (18).
14. Mokoena N, Kern A. Experiences of siblings to children with autism spectrum disorder. Front Psychiatry 2022; 13 : 959117.
15. Gandal MJ, Haney JR, Wamsley B, et al. Broad transcriptomic dysregulation occurs across the cerebral cortex in ASD. Nature 2022; 611 (7936): 532-9.
16. Chen Y, Dai J, Tang L, et al. Neuroimmune transcriptome changes in patient brains of psychiatric and neurological disorders. Mol Psychiatry 2022.
17. Menassa DA, Muntslag TAO, Martin-Estebane M, et al. The spatiotemporal dynamics of microglia across the human lifespan. Dev Cell 2022; 57 (17): 2127-39 e6.
18. Wiggins LD, Tian LH, Rubenstein E, et al. Features that best define the heterogeneity and homogeneity of autism in preschool-age children: A multisite case-control analysis replicated across two independent samples. Autism Res 2022; 15 (3): 539-50.
19. Girault JB, Donovan K, Hawks Z, et al. Infant Visual Brain Development and Inherited Genetic Liability in Autism. Am J Psychiatry 2022; 179 (8): 573-85.
20. Huang Q, Pereira AC, Velthuis H, et al. GABA(B) receptor modulation of visual sensory processing in adults with and without autism spectrum disorder. Sci Transl Med 2022; 14 (626): eabg7859.
21. Habata K, Cheong Y, Kamiya T, et al. Relationship between sensory characteristics and cortical thickness/volume in autism spectrum disorders. Transl Psychiatry 2021; 11 (1): 616.
22. Seguin D, Pac S, Wang J, et al. Amygdala subnuclei volumes and anxiety behaviors in children and adolescents with autism spectrum disorder, attention deficit hyperactivity disorder, and obsessive-compulsive disorder. Hum Brain Mapp 2022; 43 (16): 4805-16.
23. Andrews DS, Aksman L, Kerns CM, et al. Association of Amygdala Development With Different Forms of Anxiety in Autism Spectrum Disorder. Biol Psychiatry 2022; 91 (11): 977-87.
24. Lee JK, Andrews DS, Ozturk A, et al. Altered Development of Amygdala-Connected Brain Regions in Males and Females with Autism. J Neurosci 2022; 42 (31): 6145-55.
25. Pretzsch CM, Schafer T, Lombardo MV, et al. Neurobiological Correlates of Change in Adaptive Behavior in Autism. Am J Psychiatry 2022; 179 (5): 336-49.
26. Connacher R, Williams M, Prem S, et al. Autism NPCs from both idiopathic and CNV 16p11.2 deletion patients exhibit dysregulation of proliferation and mitogenic responses. Stem Cell Reports 2022; 17 (6): 1380-94.
27. Revah O, Gore F, Kelley KW, et al. Maturation and circuit integration of transplanted human cortical organoids. Nature 2022; 610 (7931): 319-26.
28. Silverman JL, Thurm A, Ethridge SB, et al. Reconsidering animal models used to study autism spectrum disorder: Current state and optimizing future. Genes Brain Behav 2022; 21 (5): e12803.
29. Antaki D, Guevara J, Maihofer AX, et al. A phenotypic spectrum of autism is attributable to the combined effects of rare variants, polygenic risk and sex. Nature Genetics 2022; 54 (9): 1284-92.
30. Warrier V, Zhang X, Reed P, et al. Genetic correlates of phenotypic heterogeneity in autism. Nature Genetics 2022; 54 (9): 1293-304.
31. Zhou A, Cao X, Mahaganapathy V, et al. Common genetic risk factors in ASD and ADHD co-occurring families. Hum Genet 2022.
32. Brownstein CA, Douard E, Mollon J, et al. Similar Rates of Deleterious Copy Number Variants in Early-Onset Psychosis and Autism Spectrum Disorder. Am J Psychiatry 2022; 179 (11): 853-61.
33. Birnbaum R, Mahjani B, Loos RJF, Sharp AJ. Clinical Characterization of Copy Number Variants Associated With Neurodevelopmental Disorders in a Large-scale Multiancestry Biobank. JAMA Psychiatry 2022; 79 (3): 250-9.
34. Shimelis H, Oetjens MT, Walsh LK, et al. Prevalence and Penetrance of Rare Pathogenic Variants in Neurodevelopmental Psychiatric Genes in a Health Care System Population. American Journal of Psychiatry 2022: appi.ajp.22010062.
35. Wang T, Kim CN, Bakken TE, et al. Integrated gene analyses of de novo variants from 46,612 trios with autism and developmental disorders. Proc Natl Acad Sci U S A 2022; 119 (46): e2203491119.
36. Murtaza N, Cheng AA, Brown CO, et al. Neuron-specific protein network mapping of autism risk genes identifies shared biological mechanisms and disease-relevant pathologies. Cell Rep 2022; 41 (8): 111678.
37. Carson L, Parlatini V, Safa T, et al. The association between early childhood onset epilepsy and attention-deficit hyperactivity disorder (ADHD) in 3237 children and adolescents with Autism Spectrum Disorder (ASD): a historical longitudinal cohort data linkage study. Eur Child Adolesc Psychiatry 2022.
38. Havdahl A, Wootton RE, Leppert B, et al. Associations Between Pregnancy-Related Predisposing Factors for Offspring Neurodevelopmental Conditions and Parental Genetic Liability to Attention-Deficit/Hyperactivity Disorder, Autism, and Schizophrenia: The Norwegian Mother, Father and Child Cohort Study (MoBa). JAMA Psychiatry 2022; 79 (8): 799-810.
39. Nudel R, Thompson WK, Borglum AD, et al. Maternal pregnancy-related infections and autism spectrum disorder-the genetic perspective. Transl Psychiatry 2022; 12 (1): 334.
40. Che X, Hornig M, Bresnahan M, et al. Maternal mid-gestational and child cord blood immune signatures are strongly associated with offspring risk of ASD. Mol Psychiatry 2022; 27 (3): 1527-41.
41. Reed ZE, Larsson H, Haworth CMA, et al. Mapping the genetic and environmental aetiology of autistic traits in Sweden and the United Kingdom. JCPP Adv 2021; 1 (3): e12039.
42. Jimenez JA, Simon JM, Hu W, et al. Developmental pyrethroid exposure and age influence phenotypes in a Chd8 haploinsufficient autism mouse model. Sci Rep 2022; 12 (1): 5555.
43. Isaksson J, Ruchkin V, Aho N, Lundin Remnelius K, Marschik PB, Bolte S. Nonshared environmental factors in the aetiology of autism and other neurodevelopmental conditions: a monozygotic co-twin control study. Mol Autism 2022; 13 (1): 8.
44. Volk HE, Ames JL, Chen A, et al. Considering Toxic Chemicals in the Etiology of Autism. Pediatrics 2022; 149 (1).
45. Wigdor EM, Weiner DJ, Grove J, et al. The female protective effect against autism spectrum disorder. Cell Genomics 2022; 2 (6): 100134.
46. Dougherty JD, Marrus N, Maloney SE, et al. Can the “female protective effect” liability threshold model explain sex differences in autism spectrum disorder? Neuron 2022; 110 (20): 3243-62.
47. Burrows CA, Grzadzinski RL, Donovan K, et al. A Data-Driven Approach in an Unbiased Sample Reveals Equivalent Sex Ratio of Autism Spectrum Disorder-Associated Impairment in Early Childhood. Biol Psychiatry 2022; 92 (8): 654-62.
48. Ross A, Grove R, McAloon J. The relationship between camouflaging and mental health in autistic children and adolescents. Autism Res 2022.
49. Saure E, Castren M, Mikkola K, Salmi J. Intellectual disabilities moderate sex/gender differences in autism spectrum disorder: a systematic review and meta-analysis. J Intellect Disabil Res 2022.
50. Floris DL, Peng H, Warrier V, et al. The Link Between Autism and Sex-Related Neuroanatomy, and Associated Cognition and Gene Expression. American Journal of Psychiatry 2022: appi.ajp.20220194.
51. Rosen V, Blank E, Lampert E, et al. Brief Report: Telehealth Satisfaction Among Caregivers of Pediatric and Adult Psychology and Psychiatry Patients with Intellectual and Developmental Disability in the Wake of Covid-19. J Autism Dev Disord 2022; 52 (12): 5253-65.
52. Talbott MR, Lang E, Avila F, Dufek S, Young G. Short report: Experiences of Caregivers Participating in a Telehealth Evaluation of Development for Infants (TEDI). J Autism Dev Disord 2022; 52 (12): 5266-73.
53. Adler EJ, Schiltz HK, Glad DM, et al. Brief Report: A Pilot Study Examining the Effects of PEERS(R) for Adolescents Telehealth for Autistic Adolescents. J Autism Dev Disord 2022; 52 (12): 5491-9.
54. Estabillo JA, Moody CT, Poulhazan SJ, Adery LH, Denluck EM, Laugeson EA. Efficacy of PEERS(R) for Adolescents via Telehealth Delivery. J Autism Dev Disord 2022; 52 (12): 5232-42.
55. Jones E, Kurman J, Delia E, et al. Parent Satisfaction With Outpatient Telemedicine Services During the COVID-19 Pandemic: A Repeated Cross-Sectional Study. Front Pediatr 2022; 10 : 908337.
56. Ferrante C, Sorgato P, Fioravanti M, et al. Supporting Caregivers Remotely During a Pandemic: Comparison of WHO Caregiver Skills Training Delivered Online Versus in Person in Public Health Settings in Italy. J Autism Dev Disord 2022: 1-20.
57. McNally Keehn R, Enneking B, Ryan T, et al. Tele-assessment of young children referred for autism spectrum disorder evaluation during COVID-19: Associations among clinical characteristics and diagnostic outcome. Autism 2022: 13623613221138642.
58. Klaiman C, White S, Richardson S, et al. Expert Clinician Certainty in Diagnosing Autism Spectrum Disorder in 16-30-Month-Olds: A Multi-site Trial Secondary Analysis. J Autism Dev Disord 2022: 1-16.
59. Reisinger DL, Hines E, Raches C, Tang Q, James C, Keehn RM. Provider and Caregiver Satisfaction with Telehealth Evaluation of Autism Spectrum Disorder in Young Children During the COVID-19 Pandemic. J Autism Dev Disord 2022; 52 (12): 5099-113.
60. Charalampopoulou M, Choi EJ, Korczak DJ, et al. Mental health profiles of autistic children and youth during the COVID-19 pandemic. Paediatr Child Health 2022; 27 (Suppl 1): S59-S65.
61. Evers K, Gijbels E, Maljaars J, et al. Mental health of autistic adults during the COVID-19 pandemic: The impact of perceived stress, intolerance of uncertainty, and coping style. Autism 2022: 13623613221119749.
62. Baribeau DA, Vigod SN, Pullenayegum E, et al. Developmental cascades between insistence on sameness behaviour and anxiety symptoms in autism spectrum disorder. Eur Child Adolesc Psychiatry 2022.
63. Stewart Campbell A, Needham BD, Meyer CR, et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial. Nature Medicine 2022; 28 (3): 528-34.
64. Mournet AM, Wilkinson E, Bal VH, Kleiman EM. A systematic review of predictors of suicidal thoughts and behaviors among autistic adults: Making the case for the role of social connection as a protective factor. Clin Psychol Rev 2022; 99 : 102235.
65. Lampinen LA, Zheng S, Taylor JL, et al. Patterns of sleep disturbances and associations with depressive symptoms in autistic young adults. Autism Res 2022; 15 (11): 2126-37.
66. Safar K, Vandewouw MM, Pang EW, et al. Shared and Distinct Patterns of Functional Connectivity to Emotional Faces in Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder Children. Front Psychol 2022; 13 : 826527.
67. Schachar RJ, Dupuis A, Arnold PD, et al. Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder: Shared or Unique Neurocognitive Profiles? Res Child Adolesc Psychopathol 2022.
68. Carter Leno V, Wright N, Pickles A, et al. Exposure to family stressful life events in autistic children: Longitudinal associations with mental health and the moderating role of cognitive flexibility. Autism 2022; 26 (7): 1656-67.
69. Lei J, Charman T, Leigh E, Russell A, Mohamed Z, Hollocks MJ. Examining the relationship between cognitive inflexibility and internalizing and externalizing symptoms in autistic children and adolescents: A systematic review and meta-analysis. Autism Res 2022; 15 (12): 2265-95.
70. Forbes G, Kent R, Charman T, Baird G, Pickles A, Simonoff E. How do autistic people fare in adult life and can we predict it from childhood? Autism Research 2022; n/a (n/a).
71. Pham HH, Sandberg N, Trinkl J, Thayer J. Racial and Ethnic Differences in Rates and Age of Diagnosis of Autism Spectrum Disorder. JAMA Netw Open 2022; 5 (10): e2239604.
72. Kalb LG, Singh V, Hong JS, et al. Analysis of Race and Sex Bias in the Autism Diagnostic Observation Schedule (ADOS-2). JAMA Netw Open 2022; 5 (4): e229498.
73. DuBay M. Cultural Adaptations to Parent-Mediated Autism Spectrum Disorder Interventions for Latin American Families: A Scoping Review. Am J Speech Lang Pathol 2022; 31 (3): 1517-34.
74. Vanegas SB, Duenas AD, Kunze M, Xu Y. Adapting parent-focused interventions for diverse caregivers of children with intellectual and developmental disabilities: Lessons learned during global crises. J Policy Pract Intellect Disabil 2022; na : 1-13.
75. Steinbrenner JR, McIntyre N, Rentschler LF, et al. Patterns in reporting and participant inclusion related to race and ethnicity in autism intervention literature: Data from a large-scale systematic review of evidence-based practices. Autism 2022; 26 (8): 2026-40.
76. Williams EG, Smith MJ, Boyd B. Perspective: The role of diversity advisory boards in autism research. Autism 2022: 13623613221133633.
77. Reetzke R, Singh V, Hong JS, et al. Profiles and correlates of language and social communication differences among young autistic children. Front Psychol 2022; 13 : 936392.
78. Buijsman R, Begeer S, Scheeren AM. ‘Autistic person’ or ‘person with autism’? Person-first language preference in Dutch adults with autism and parents. Autism 2022: 13623613221117914.
79. Monk R, Whitehouse AJO, Waddington H. The use of language in autism research. Trends Neurosci 2022; 45 (11): 791-3.
80. Bury SM, Jellett R, Haschek A, Wenzel M, Hedley D, Spoor JR. Understanding language preference: Autism knowledge, experience of stigma and autism identity. Autism 2022: 13623613221142383.
81. Keating CT, Hickman L, Leung J, et al. Autism-related language preferences of English-speaking individuals across the globe: A mixed methods investigation. Autism Res 2022.
82. Singer A, Lutz A, Escher J, Halladay A. A full semantic toolbox is essential for autism research and practice to thrive. Autism Res 2022.
Sign up to receive resources
- Open access
- Published: 18 December 2024
Exploring autism spectrum disorder and co-occurring trait associations to elucidate multivariate genetic mechanisms and insights
- Karoliina Salenius 1 na1 ,
- Niina Väljä 1 na1 ,
- Sini Thusberg 1 ,
- Francois Iris 3 ,
- Christine Ladd-Acosta 4 ,
- Christophe Roos 5 ,
- Matti Nykter 1 , 6 ,
- Alessio Fasano 7 , 8 ,
- Reija Autio 9 na1 ,
- Jake Lin 1 , 10 na1 on behalf of
the GEMMA study
BMC Psychiatry volume 24 , Article number: 934 ( 2024 ) Cite this article
84 Accesses
Metrics details
Autism spectrum disorder (ASD) is a partially heritable neurodevelopmental trait, and people with ASD may also have other co-occurring trait such as ADHD, anxiety disorders, depression, mental health issues, learning difficulty, physical health traits and communication challenges. The concomitant development of ASD and other neurological traits is assumed to result from a complex interplay between genetics and the environment. However, only a limited number of studies have performed multivariate genome-wide association studies (GWAS) for ASD.
We conducted to-date the largest multivariate GWAS on ASD and 8 ASD co-occurring traits (ADHD, ADHD childhood, anxiety stress (ASDR), bipolar (BIP), disruptive behaviour (DBD), educational attainment (EA), major depression, and schizophrenia (SCZ)) using summary statistics from leading studies. Multivariate associations and central traits were further identified. Subsequently, colocalization and Mendelian randomization (MR) analysis were performed on the associations identified with the central traits containing ASD. To further validate our findings, pathway and quantified trait loci (QTL) resources as well as independent datasets consisting of 112 (45 probands) whole genome sequence data from the GEMMA project were utilized.
Multivariate GWAS resulted in 637 significant associations ( p < 5e-8), among which 322 are reported for the first time for any trait. 37 SNPs were identified to contain ASD and one or more traits in their central trait set, including variants mapped to known SFARI ASD genes MAPT , CADPS and NEGR1 as well as novel ASD genes KANSL1 , NSF and NTM , associated with immune response, synaptic transmission, and neurite growth respectively. Mendelian randomization analyses found that genetic liability for ADHD childhood, ASRD and DBT has causal effects on the risk of ASD while genetic liability for ASD has causal effects on the risk of ADHD, ADHD childhood, BIP, WA, MDD and SCZ. Frequency differences of SNPs found in NTM and CADPS genes, respectively associated with neurite growth and neural/endocrine calcium regulation, were found between GEMMA ASD probands and controls. Pathway, QTL and cell type enrichment implicated microbiome, enteric inflammation, and central nervous system enrichments.

Conclusions
Our study, combining multivariate GWAS with systematic decomposition, identified novel genetic associations related to ASD and ASD co-occurring driver traits. Statistical tests were applied to discern evidence for shared and interpretable liability between ASD and co-occurring traits. These findings expand upon the current understanding of the complex genetics regulating ASD and reveal insights of neuronal brain disruptions potentially driving development and manifestation.
Multivariate GWAS resulted in 637 significant ASD associations ( p < 5e-8), among which 322 are reported for the first time.
The novel associations mapped to known SFARI ASD genes CADPS , MAPT and NEGR1 and novel ASD genes KANSL1 , NSF and NTM , associated with immune response, synaptic transmission, and neurite growth, potentially driving the gut brain-barrier hypothesis underpinning ASD development.
CuONPs induce co-occurrence of autophagy activation and autophagic flux blockade.
Mendelian randomization analyses found that genetic liability for ASRD and DBT have causal effects on the risk of ASD while genetic liability for ASD have causal effects on the risk of ADHD, BIP, WA, MDD and SCZ. Bidirectional genetic liability causal effects were confirmed between ASD and ADHD childhood.
Peer Review reports
Introduction
ASD spectrum disorders (ASD) is an umbrella term for a group of heterogeneous neurodevelopmental traits that manifest in early childhood. ASD is a complex disorder with both genetic and environmental risk factors [ 10 , 30 , 45 ]. The diagnosis of ASD is based on its key characteristics including difficulties in social communication and interaction, restricted and repetitive behaviors, hyperactivity and divergent responses to sensory inputs. The most common co-occurring traits in autistic persons are attention deficit hyperactivity disorder (ADHD), ADHD childhood, anxiety, bipolar (BP), depression, epilepsy, obsessive compulsive disorders (OCD) and stress related traits, all of which share overlapping diagnostic attributes and challenging symptoms with ASD [ 30 , 57 ]. According to US data, autistic children tend to fare less well in educational attainment (EA) and about one in three have a reduced intellectual ability, as defined by intelligence quotient (IQ less than 70) [ 4 , 68 ]. Some children with ASD having higher IQ scores also comparatively experience harder academic struggles due to co-occurring traits and difficulties in social interactions [ 3 ].
Together with recent advances in genomics technology and pivotal support from the engaged ASD community, 1,162 genes are currently implicated with ASD development and these are curated in the SFARI [ 2 , 19 , 52 ] gene module. These genes, with varying degrees of effect, are scored using the Evaluation of ASD Gene Link Evidence (EAGLE) framework [ 61 ]. Surprisingly, while it is known that common variants contribute to most of the genetic background [ 18 ], only a few robust genetic associations have been recently reported. Most of these are attributed to the landmark study conducted by Grove and colleagues, employing a large Danish cohort with 18,381 ASD cases and 27,969 controls, where 12 significant variant associations were reported [ 19 ].
Given that there is overlap in symptoms between ASD and ADHD, recent genetics studies found shared genetic factors underlying ASD and ADHD [ 40 , 41 , 50 ], with partial concordance between bidirectional colocalization single nucleotide variants (SNPs). However, these studies were limited to general ADHD (onset age 10+), and not childhood ADHD. Astoundingly many (47% median) autistic children have reported one or more gastrointestinal (GI) symptoms [ 5 ]. Recently, there have been promising results that link microbiome disruption and diversity [ 44 ] as a novel contributing factor to ASD. While Grove and colleagues found that 7 of the 12 ASD SNP associations have similar significance towards EA and psychosis traits depression and schizophrenia [ 19 ], still little is known concerning the joint liability and the shared genetic mechanisms between ASD and ASD co-occurring traits including ADHD, ADHD childhood, anxiety-stress related disorder (ASRD), bipolar, disruptive behavior disorder (DBD), EA, epilepsy, inflammatory bowel disease (IBD), major depression, obsessive compulsive disorder (OCD) and schizophrenia (SCZ). Respectively, the 11 co-occurring trait summary statistics are retrieved from large reputable cohorts, listed in Table 1 and Supplementary Table 1.
To attenuate the genetic knowledge gaps in ASD and expand the exploration of potential shared co-occurring trait genetic associations, this study performed multivariate genome-wide association study (GWAS) with summary statistics from ASD and 11 co-occurring traits from large reputable cohorts. To achieve this, colocalization (coloc) was systematically applied to test the robustness between the shared variants and traits [ 75 ]. Mendelian randomisation (MR) was further applied, using the multivariate variants and the essential traits, to assess liability relationships between ASD and the selected co-occurring traits [ 6 , 55 ]. This study seeks to further clarify functional, regulatory and tissue type differentiation with enrichment and integration of quantified trait loci (QTL) while validating our key findings with independently sequenced genomes from the GEMMA cohort [ 70 ].
Methods and materials
GWAS summary statistics for ASD and ADHD were collected from the Psychiatric Genomics Consortium (PGC) and iPSYCH [ 49 , 65 ] studies. Education attainment [ 47 ] summary file was collected from the Social Science Genetic Association Consortium (SSGAC). Additional ASD co-occurring traits, selected based on LDSC (LD Score Regression) genetic correlation ( p -value < 0.05) with ASD, include ADHD childhood, bipolar (BP), anxiety-stress disorder (ASRD), disruptive behaviour (DBD), major depression (MDD) and schizophrenia (SCZ), with sample sizes ranging from 31,890 − 765,283 are shown in Table 1 (additional details including doi references listed Supplementary Table 1). To estimate potential sample overlaps, pairwise LDSC intercepts with ASD are calculated and reported in Supplementary Table 1. Summary statistics are joined, yielding 4,525,476 SNPs, and applied in a multivariate GWAS setting. Follow-up analysis includes decomposition aiming to detect the most important traits while colocalization and Mendelian randomisation analysis are conducted to explore shared liability as shown in Fig. 1 .
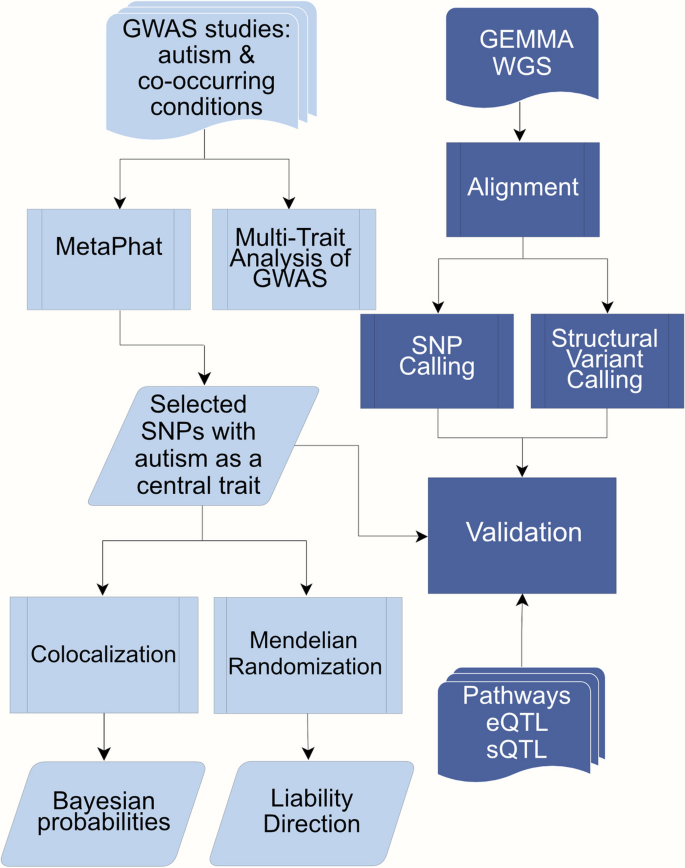
Workflow for the analyses conducted in the study. Multivariate GWAS was performed on selected GWAS studies including ASD and 8 co-occurring traits: ADHD, ADHD childhood, bipolar, anxiety, disruptive behaviour, educational attainment, major depression and schizophrenia. 37 SNPs were selected and evaluated with Colocalization and Mendelian Randomization. Further validation of these SNPs utilized pathway and EBI eQTL/sQTL catalogs as well as the GEMMA -study. The GEMMA whole genome sequencing (WGS) processing included variant calling to infer structural and single nucleotide variants (SVs and SNVs) present in the samples
Multivariate GWAS and determination of central traits
Multivariate GWAS on ASD and ASD co-occurring traits were performed using metaPhat/metaCCA software that performs multivariate analysis by implementing Canonical Correlation Analysis (CCA) for a set of univariate GWAS summary statistics [ 12 , 36 , 58 ]. The objective of metaCCA is to find the optimal genetic effect combination that is maximally correlated with a linear combination of the trait variables. ASD multivariate central traits are identified by MetaPhat decomposition based on iterative tracing of p -values (p) from trait subsets (relative to 5e-8) and Bayesian Information Criterion (BIC) [ 62 ] representing model fit. Essentially, driver trait(s) are the subsets of the multivariate association that drives the p-value, and without the drivers, the multivariate association is no longer significant ( p > 5e-8). Similarly, as the decomposition processing is exhaustive (iterates from k to 1), an optimal subset is identified by comparing BIC values [ 36 ]. For simplicity, the central traits are the union of the driver and optimal BIC traits. Multi-Trait Analysis of GWAS (MTAG) [ 71 ], a high performance multivariate-GWAS that addresses sample overlap, is additionally performed for validation.
Genetic annotations, pathway enrichment and validation
SFARI Base Gene resource, GeneCards and GWAS catalog were used to assess the novelty of variants and genes associated with ASD [ 2 , 39 , 60 ]. snpXplorer was applied towards SNP annotation [ 69 ]. Reactome and WikiPathway databases pathway enrichments were evaluated with the Enrichr tool [ 31 ]. Human organ and cell type systems enrichment analysis, encompassing 1,466 tissue-cell type and single-cell RNAseq panels, was conducted using WebCSEA [ 13 , 33 ]. eQTL and sQTL were assessed within the QTL catalog, via FIVEx portal [ 32 ].
Colocalization analyses
Colocalization was performed for the selected multivariate ASD SNPs to assess if the associated variants in the locus are shared genetically between ASD and the 8 co-occurring related ASD traits to account for erroneous results that may follow from analyzing individual SNPs. Errors can occur when a SNP associated with trait 1 and trait 2 are in linkage disequilibrium (LD). The analyses were performed using the R package coloc [ 20 , 33 ].
The colocalization analysis was conducted using the absolute base factor colocalization method (coloc.abf), which is a Bayesian colocalization analysis method. A region size window of 100KB (± 50 KB flanking the SNP position) was selected to comprehensively span potential LD and regulatory elements [ 53 ]. The different hypotheses tested include: H0 (no liable variant), H1 (liable variant only for trait 1), H2 (liable variant only for trait 2), H3 (two separate liable variants), H4 (common liable variant shared between the traits). As recommended [ 74 ], default setting prior probability thresholds were applied: 1e-4 for H1, H2 and H3 and 1e-5 for H4 while posterior probability (H4 > 90%) is conservatively applied to estimate shared liability.
Mendelian randomization analyses
Mendelian Randomization analyses (MR) was conducted on the selected multivariate GWAS SNPs based on their assigned central traits, to explore the liability, direction and independent (reverse causation) relationships between ASD and its related traits [ 51 ]. Instrumental strengths, approximated with F1 score > 10, were calculated using SNP effect and standard error values [6, 49]. To account for the potential biases due to participant overlap between cohorts, the lower bound (95% confidence interval) of the F1 was calculated [ 9 ]. The analyses were performed using the platform TwoSampleMR [ 6 ].
Whole genome sequencing
The results were validated using yet unpublished data from the EU Horizon2020 GEMMA research project with genotype variant calls in 112 (49% female) WGS samples with 45 ASD probands (42% female) from the GEMMA prospective cohort [ 70 ]. These samples, assayed on whole blood and collected during enrollment, were sequenced with 30-40X coverage on Illumina NovaSeq 6000 platform. Data was aligned to GRCh38 reference genome using bwa mem v0.7.17 [ 34 ] and reads were sorted and duplicates marked with samtools v1.12 [ 35 ]. Quality control was performed with omnomicsQ -software [ 20 ]. For variant calling DeepVariant v1.4.0 [ 54 ] was utilized and variants were annotated with Variant Effect Predictor [ 43 ] version 112.0.
Statistical analysis
All statistical analyses were performed using R 4.2.2 software and available as R markdown results in the github project ( https://github.com/jakelin212/mvasd_gwas ). Genome-wide association is called on the standard and strict p-value threshold of 5e-8 (-log10 7.3), to account for multiple testing based on the assumption of about 1-million independent tests [ 56 ]. To assess SNP allele proportional differences for validation, the phi coefficient is computed, and statistical significance was determined using Chi-square test. Fisher’s exact test was used when Chi-square assumptions were not met. Bonferroni correction is assessed to account for multiple testing of the multivariate GWAS involving 9 traits ( p < 5.5e-9; -log10(p) > 8.25).
GWAS summary statistics
GWAS summary statistics for ASD and ADHD were collected from the PGC and iPSYCH [ 49 , 65 ] studies. Education attainment [ 47 ] summary file was collected from the Social Science Genetic Association Consortium (SSGAC). Altogether, using summary statistics, 11 ASD co-occurring traits were assessed for genetic correlation with the landmark ASD study [ 19 ], the largest genetic correlation values, as computed by LDSC [ 8 ], were between ASD and ADHD (rg = 0.535), followed by MDD (rg = 0.505) and ADHD childhood (rg = 0.478). Shown in Table 1 below, 8 traits are shown to be genetically correlated with ASD ( p < 0.05) and additional details of all traits are shown in Supplementary Table 1.
Multivariate ASD central trait SNPs, pathway and organ tissue enrichment
Multivariate GWAS was performed with ASD together with its genetically correlated traits, ADHD, ADHD childhood, ASRD, bipolar, DBD, EA, MDD, and SCZ (Table 1 ) and 637 ( p < 5e-08) SNP associations were found, including 322 variants that are reported for the first time for any trait (Supplementary Table 6) according to GWAS catalog. Two associations (rs2388334 and rs1452075) intersected with the twelve associations identified in the landmark common genetic variants of ASD study [ 19 ]. When assessed at the gene level, all 12 were concordant (as indicated in STable 6). Decomposition implemented in MetaPhat, using stepwise tracing of p -value and Bayesian information criteria (BIC) contributions [ 36 , 62 ], identified 37 ASD central trait SNPs where 16 were identified with multivariate GWAS approach (all SNPs p < 5.5e-09; min (-log10(p) 8.67), listed in Supplementary Table 2). These 37 multivariate ASD SNPs, 17 of which had previously been reported in existing GWAS studies, mapped to 35 genes (Table 2 ) and confirmed that 8/35 ( ARHGAP32 , CADPS , CUL3 , KANSL1 , MACROD2 , MAPT , MSRA and NEGR1 ) are known curated SFARI genes, with ASD susceptibility EAGLE scores < = 3 (indicating limited evidence) [ 61 ]. The variant rs538628 within the NSF gene, a regulator of AMPA receptor endocytosis and critical for mediating glutamatergic synaptic transmission [ 25 ], along with the variant rs62061734 in the MAPT gene, are identified to associate with the optimal central traits of ASD, EA and SCZ ( MAPT variant rs62061734 p = 3.98e-31, NSF variant rs538628 p = 1.99e-27, Supplementary Table 2, trace plots are provided in supplementary data). Notably, NSF was previously implicated only in mouse models exhibiting ASD-like behaviors [ 76 ]. Shown in the same table, MTAG [ 71 ] multivariate GWAS validation was performed to address iPSYCH cohort sample overlaps between ADHD and ASD [ 40 , 41 ] subjects where similar results were found ( MAPT variant rs62061734 p = 1.99e-20, NSF variant rs538628 p = 5.37e-18).
Shown in Supplementary Table 7, Fig. 2 e and Supplementary Fig. 3, pathway enrichment using the 35 associated genes was performed with Enrichr [ 31 ]. Nervous systems development (GO:0007399) was found to be the most significant ( p = 1.73e-08) while neural and microtubule structural related pathway hits from Reactome [ 16 ] and WikiPathways [ 46 ] featured pathways were Inclusion Body Myositis ( MAPT and PSEN1 , p = 1.27e-04) and COPII-mediated Vesicle Transport ( NSF and SERPINA1 , p = 4.69e-03). Enrichment analysis was conducted using the WebCSEA tool, which identified statistically significant associations (Fig. 2 f, p < 1e-03) with the following human organ systems: digestive, nervous, sensory, lymphatic, and respiratory. As shown in Supplementary Fig. 4, the most enriched tissue types are related to cerebrum, cortex, intestine and blood related components discerned from 1,355 tissue-type (TS) as well as data from the human brain single cell project [ 33 ].
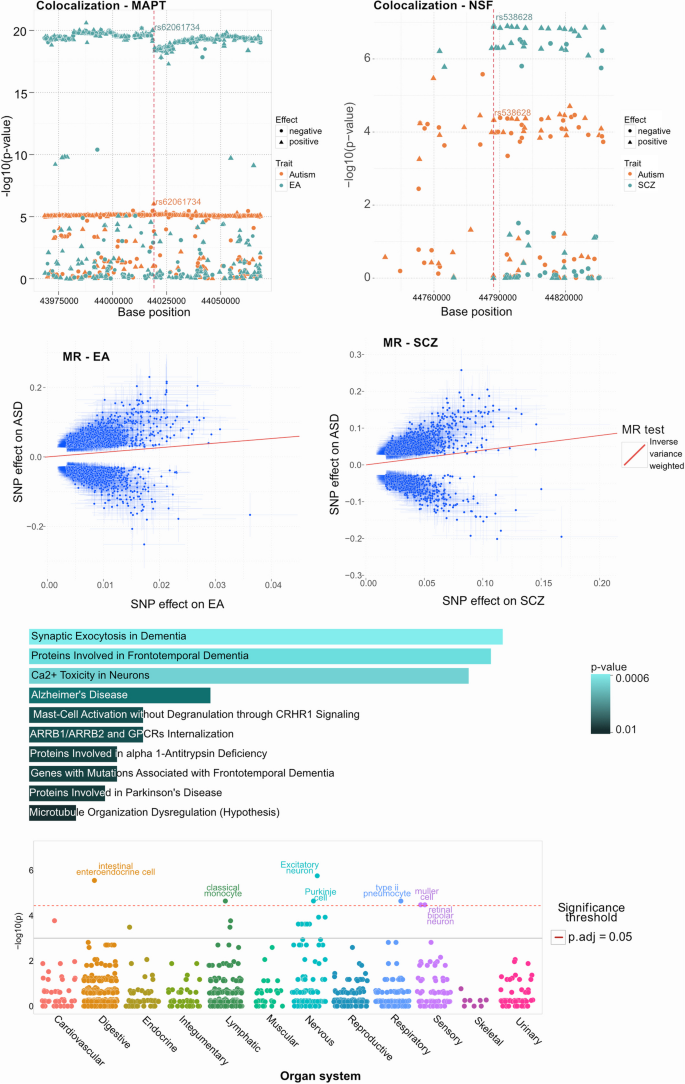
Results from the post GWAS analysis of the 37 selected SNPs. a,b ) Colocalization processing using the original summary statistics of ASD and EA for (a) rs62061734 ( MAPT , failed colocalization with H4 probability 8.19%, p = 0.09), ASD and NSF for (b) rs538628 ( NSF , SCZ passed colocalization with H4 probability 94%, p = 1.1e-05), depicting supporting regional SNPs (x-axis) and their negative log10 p -value (y-axis) and effect direction (circles negative, triangles positive). c,d ) Mendelian randomization (MR) results using inverse variance weighted (IVW) -method for association of ASD SNP effects (y-axis) and c) EA and d) SCZ effects (x-axis). e ) Pathway analysis for the genes associated with the selected SNPs shows enrichment in processes related to neurons using Reactome database. The length of the bar represents the significance of that specific gene-set or pathway and the color indicates the significance of the pathway. Details of the pathways and genes with their associated p-values are listed in Supplementary Table 8. f ) Organ system enrichment was applied using WebCSEA, using the selected 37 multivariate gene associations and found enrichment ( p < 1e-03) with the ASD relevant digestive, nervous and sensory organ systems as well as lymphatic and respiratory systems
Colocalization analysis was conducted on the 37 multivariate SNP associations identified to contain ASD as a central trait. The comparative analysis was performed on the relevant mapped gene window, from start to end while adding 25 KBs on both ends to cover regulating and promoter regional elements. For the two SNPs that did not map to a gene, the window size used for the colocalization analysis was 100 KB (± 50 KB), estimated and derived from the gene median length of 24KB [ 17 ]. Additional information concerning the number of regional LD adjusted SNPs applied to the colocalization test is shown in Supplementary Table 3.
A total of 19/37 SNPs showed strong evidence for a common liability variant with ASD (H4 > 90%, details shown in Supplementary Table 3) and the traits having common ASD liable variants included EA (9), SCZ (6), BP (2), ADHD (1) and ASRD (1). Notably, SNP rs62061734, mapping to the MAPT gene and rs538628, mapping to the NSF gene had H4 of 99% for EA and SCZ, respectively (shown in Fig. 2 a-b) while SNP rs568828, mapping to the NTM gene had H4 of 99% for SCZ and ADHD (Supplementary Table 2).
Mendelian randomization analysis was conducted for the 8 traits genetically correlated (Table 1 ) with ASD. The lead SNPs, with F1 scores > 25 (listed in Supplementary Table 4, where > 10 is considered strong [ 48 ] were found to lend significantly increase probability of ASD ( p < 0.001 both Inverse Variance Weighted (IVW)-method and MR-Egger (EA and SCZ are shown in Fig. 2 c-d), accounting for horizontal pleiotropy and multiple testing with Bonferroni correction of 8 traits). Based on TwoSampleMR Steiger [ 22 ] test for directionality and shown in supplementary Table 4 A, genetic liability to ADHD childhood ( p < 2.44e-116), ASDR ( p < 9.08e-166) and DBD ( p < 1.20e-45) were found to have causal effects on the risk of ASD. Shown in Supplementary Table 4B, genetic liability to ASD ( p < 4.1e-115) were found to have causal effects on the risk of ADHD, ADHD childhood, BIP, EA, MDD and SCZ. The related MR results adhere to the MR-STROBE guidelines [ 64 ].
To assess the impact of the reported multivariate associations on expression (eQTL) and splicing regulatory quantitative trait loci across tissues, the majority (22/37 eQTL, 24/37 sQTL, details listed in Supplementary Table 9) of the associations found are cited in the EBI QTL Catalog [ 28 ] where they associate (adjusted p < 0.05) with adipose, brain and neuron tissues. Furthermore, filtering on GeneCards [ 60 ] curations, the presented ASD central genes are enriched with systems related to gut, microbiome, intestinal immune, enteric nervous and central nervous systems (Supplementary Table 5).
Additionally, the distribution of these ASD-central trait related SNPs in 112 (49% females; 45 ASD probands (42% females) prospective from-birth GEMMA [ 70 ] cohort participants was investigated. SNP distribution differences were for variant rs568828, mapped to NTM and rs62243489, mapped to CADPS . The NTM gene, previously associated with emotional learning deficits in murine models [ 42 ], encodes neurotrimin, while CADPS encodes a neural/endocrine-specific membrane protein regulating calcium. The NTM SNP (rs568828) was present in 42 of 45 probands (92%) compared to 100% of controls (67 of 67). In contrast, CADPS SNP (rs62243489) was found in 19/67 controls (28%) and enriched in 21/45 probands (47%). As listed in Supplementary Table 8, the phi coefficient for NTM between probands and controls was 0.2 ( p = 0.062), while for CADPS , it was − 0.19 ( p = 0.047). When stratified by sex, the phi coefficient for NTM in males was 0.15 ( p = 0.456) and 0.27 ( p = 0.040) in females. For CADPS , the phi coefficients were − 0.18 ( p = 0.182) in males and − 0.20 ( p = 0.140) in females. Notably, the NEGR1 gene (variant rs6699841), involved in neuron growth regulation, showed a phi coefficient of −0.27 ( p = 0.040) in males (24/26 cases; 22/31 controls), while in females, the coefficient was 0.26 ( p = 0.084; 12/19 cases; 31/36 controls). For the variant of NEGR1 , the opposing phi directions between sexes resulted in a phi coefficient of −0.01 ( p = 0.908) in the full dataset. In addition, logistic regression was performed for the specific variants of NTM , CADPS and NEGR1 . The results were not significant for the full cohort (adjusted for sex) or in models stratified by sex.
Using multivariate statistical learning approaches, this study constitutes the largest and most comprehensive genetically correlated multi-trait GWAS analysis with summary statistics performed on ASD and its genetically correlated traits; ADHD, ADHD childhood, ASRD, bipolar, DBD, EA, MDD, and SCZ to explore the underpinnings driving the complexities in ASD. 37 associations containing ASD as a central trait were discovered, with 16 of these associations were detected only due to the increased statistical power of this multivariate GWAS analysis (lowest univariate summary statistics p-value from all traits > 5e-08, and 12/16 confirmed with the MTAG tool [ 71 ], Supplementary Table 2). Interestingly, a previous study using electronic health records of covering nearly 5,000 ASD cases found three subclusters of comorbidity trajectories, first characterized by seizures, then auditory disorders/infections and the third cluster by psychiatric disorders. Due to the complexity of ASD development, a fourth group was described as could not be further resolved. The presented subclusters potentially align well with our ASD central trait sets pertaining to SCZ signals with seizures and psychiatric disorders such as ADHD and intellectual development underpinning EA [ 15 ]. Enrichment analysis confirmed that the multivariate ASD association results are related to neuron and gut tissues and developmental pathways as well as inflammation and microbiome domains, further underscoring the intersection of genome and microbiome as well as supportive of the gut-brain axis hypothesis associated to ASD [ 11 , 44 ]. Surprisingly, genetic correlation performed on LDSC indicated that ASD and IBD are not related (Supplementary Table S1 , rg = −0.059; p = 0.44), a recent report highlighted potential evidence for comorbidity between parental, particularly maternal preexisting IBD onsets and their children developing ASD [ 59 ]. Using the multivariate ASD central trait gene sets, based on comprehensive human tissue cell type and single cell data [ 13 , 33 ] analysis, enrichments were detected with digestive, nervous, and sensory organ systems (Fig. 2 f). At the tissue cell type level and further supporting the gut-brain axis and blood brain barrier, the analysis detected enriched ASD relevant signals related to brain, adipose and gut eQTL/sQTL (Supplementary Table 9) tissue panels.
Overall, the identified ASD traits passed MR with strong F1 measures and significantly contributed to improve the future construction of meta psychiatric based ASD polygenic scores [ 27 ], shown to improve prediction relative to standard PRS in other complex traits such as coronary heart disease and type 2 diabetes [ 37 , 67 ]. The MR results were consistent after calculation of lower bound F1 (all scores > 25, Supplementary Table 4), computed to consider potential biases from cohort sample overlaps [ 9 ]. MR Steiger tests for directionality revealed that genetic liability to ASDR and DBD were found to have causal effects on the risk of ASD. These multivariate ASD associations mapped to genes, including MAPT and NSF which are known to involved in biological pathways linked to neural disorders such as infantile epilepsy [ 66 ] and Parkinson’s Disease [ 7 , 14 ]. Interestingly, colocalization tests for the MAPT region indicated shared genetic risk between only EA and ASD (H4 99%), while that for the NSF gene did not associate with EA, instead associated with SCZ (H4 94%), suggesting intra region heterogeneity that demands future investigation. With respect to ASD, the KANSL1 , BNIP3 , CADPS and NEGR1 genes have been implicated with immune and microbiome features [ 11 ] and behavioral developments [ 63 ]. Similarly, a recent study from Arenella and colleagues reported genetic factors between ASD and various immune phenotypes including KANSL1 associating with lymphocyte counts as well as MAPT associating with eosinophil counts, further supporting the role of the inflammation pathway in ASD development [ 1 ].
The most common traits in our set of 37 associations that passed colocalization with ASD were EA (9), SCZ (6) and BP (2). It is known that the diagnosis for ASD and ADHD, particularly ADHD manifestation in young children, is similar with symptomatic issues concerning hyperactivity and attention span [ 29 ]. While a previous study has performed comparison of genetic and functional enrichment of associations between ASD and ADHD [ 50 ] GWAS resources, this study further complements their results by inclusion of other ASD co-occurring traits, including ADHD and ADHD childhood as well as EA. Interestingly, ASD and ADHD have both been linked with dysbiosis disruption in microbiome composition and function, gastrointestinal and bowel habits issues [ 44 ].
As part of validation, clustering and distribution proportion differences based on the ASD identified SNP associations were detected between probands and non-autistic subjects on genomes from the GEMMA cohort [ 70 ]. Our validation results were performed on the (112, 45 ASD probands) samples currently available in GEMMA. Notably, NEGR1 (rs1432639), a neuronal growth regulator known to associate with migraine, depression and seizures [ 24 , 26 , 73 ], the significant phi coefficients were negative for males and positive for females. Interestingly, a previous study pertaining to prenatal stress found increased NEGR1 expression in the hippocampus of female rats but not in males [ 72 ]. To improve on the specificity and clinical value of the identified traits, a follow-up application of MR with specific expression/protein quantitative loci (tissue/cell type e/pQTL as applied in T1D drug candidate discovery [ 21 ] with genes such as CADPS , NTM and NEGR1 could further reveal molecular and translational insights towards ASD heterogeneity including the high vulnerability subgroup characterized by seizures [ 15 ]. While the validation statistical power was limited by the relatively small sample size, nevertheless the independent and deep sequencing data has allowed the harvesting of interesting observations concerning the distribution of ASD-central trait associations in probands as compared to controls. In addition, the GEMMA validation results should be taken with caution as the population structures (PCs) were not included as covariates due to availability. The upcoming release of additional omics data from GEMMA and other studies, including longitudinal microbiome, metabolome, and methylation datasets, will significantly increase statistical power and enable more detailed temporal analyses. The data will help confirm molecular changes along the gut-brain axis, shedding light on the genetic patterns that contribute to the heterogeneity, development, and comorbidities of ASD. Another limitation of our multi-trait GWAS is that the selection of ASD co-occurring traits is not exhaustive; given the complexity of ASD development, there may be other genetically correlated traits that have not yet been tested at the appropriate population level, warranting consideration and inclusion in future studies. The MR Steiger results on causality need to be taken with caution as unmeasured confounding effects may distort the exposure genetic liability relative to the outcome [ 38 ].
Our study represents the largest multivariate GWAS on ASD to date, combining ASD with eight genetically correlated trait GWAS summaries. We performed systematic decomposition to identify novel genetic associations related to ASD and ASD co-occurring traits. Mendelian randomization testing revealed that genetic liability for ADHD childhood, ASRD and DBD has causal effects on the risk of ASD. Colocalization analysis further confirmed shared genetic risks with ASD, showing enrichment patterns in brain tissues and cell types associated with neurodevelopment, and lending additional support to the gut-brain axis hypothesis.
Data availability
All data generated or analyzed during the study are included and additionally available upon request. For scripts, please see: https://github.com/jakelin212/mvasd_gwas
Arenella M, Fanelli G, Kiemeney LA, Grainne McAlonan DG, Murphy, and Janita Bralten., Brain., behavior, & immunity - health vol. 34 100698. 3 Nov, https://doi.org/10.1016/j.bbih.2023.100698
Arpi MN, Torres, Ian Simpson T. SFARI genes and where to find them; modelling Autism Spectrum Disorder Specific Gene expression dysregulation with RNA-Seq Data. Sci Rep. 2022;12(1):10158.
Article CAS PubMed PubMed Central Google Scholar
Ashburner J, Ziviani J, and Sylvia Rodger. Surviving in the mainstream: capacity of children with Autism Spectrum disorders to perform academically and regulate their emotions and behavior at School. Res Autism Spectr Disorders. 2010;4(1):18–27.
Article Google Scholar
Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, et al. Prevalence of Autism Spectrum Disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. Morbidity Mortal Wkly Rep Surveillance Summaries. 2018;67(6):1–23.
Google Scholar
Boorstein HC. Gastrointestinal and feeding problems in Young children: a comparison between Autism Spectrum disorders and other Developmental delays. University of Connecticut; 2008.
Bowden J, Smith GD, and Stephen Burgess. Mendelian randomization with Invalid instruments: Effect Estimation and Bias Detection through Egger Regression. Int J Epidemiol. 2015;44(2):512–25.
Article PubMed PubMed Central Google Scholar
Brion JP, Octave JN, Couck AM. Distribution of the Phosphorylated Microtubule-Associated protein tau in developing cortical neurons. Neuroscience. 1994;63(3):895–909.
Article CAS PubMed Google Scholar
Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Patterson N, Daly MJ, Price AL, Neale BM. LD score regression distinguishes confounding from polygenicity in genome-wide Association studies. Nat Genet. 2015;47(3):291–95.
Burgess S, Davies NM, and Simon G. Thompson. Bias due to participant overlap in two-sample mendelian randomization: Burgess Et Al. Genet Epidemiol. 2016;40(7):597–608.
Chaste P, and Marion Leboyer. Autism risk factors: genes, Environment, and gene-environment interactions. Dialog Clin Neurosci. 2012;14(3):281–92.
Cheng L, Wu H, Chen Z, Hao H, and Xiao Zheng. Gut microbiome at the crossroad of genetic variants and Behavior disorders. Gut Microbes. 2023;15(1):2201156.
Cichonska A, Rousu J, Marttinen P, Kangas AJ, Soininen P, Lehtimäki T, Raitakari OT, et al. metaCCA: Summary statistics-based multivariate Meta-analysis of Genome-Wide Association Studies Using Canonical Correlation Analysis. Bioinformatics. 2016;32(13):1981–89.
Dai Y, Hu R, Liu A, et al. WebCSEA: web-based cell-type-specific enrichment analysis of genes. Nucleic Acids Res. 2022;50(W1):W782–90. https://doi.org/10.1093/nar/gkac392 .
Derkinderen P, Rolli-Derkinderen M, Chapelet G, Neunlist M, and Wendy Noble. Tau in the gut, does it really Matter? J Neurochem. 2021;158(2):94–104.
Doshi-Velez, Finale Y, Ge, and Isaac Kohane. Comorbidity clusters in Autism Spectrum disorders: an Electronic Health Record Time-Series Analysis. Pediatrics. 2014;133(1):e54–63.
Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, Haw R, et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018;46(D1):D649–55.
Fuchs G, Voichek Y, Benjamin S, Gilad S, Amit I, and Moshe Oren. 4sUDRB-Seq: measuring Genomewide Transcriptional Elongation Rates and initiation frequencies within cells. Genome Biol. 2014;15(5):R69.
Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Milind Mahajan, et al. Most genetic risk for Autism resides with common variation. Nat Genet. 2014;46(8):881–85.
Grove J, Ripke S, Als TD, Manuel Mattheisen RK, Walters H, Won J Pallesen, et al. Identification of Common Genetic Risk variants for Autism Spectrum Disorder. Nat Genet. 2019;51(3):431–44.
Gutowska-Ding M, Weronika ZC, Deans C, Roos J, Matilainen F, Khawaja K, Brügger JW, Ahn C, Boustred, and Simon J. Patton. One byte at a time: evidencing the quality of clinical service next-generation sequencing for germline and somatic variants. Eur J Hum Genetics: EJHG. 2020;28(2):202–12.
Article PubMed Google Scholar
Heikkilä TE, Emilia K, Kaiser J, Lin D, Gill JJ, Koskenniemi. and Ville Karhunen. 2024. Genetic Evidence for Efficacy of Targeting IL-2, IL-6 and TYK2 Signalling in the Prevention of Type 1 Diabetes: A Mendelian Randomisation Study. Diabetologia, September. https://doi.org/10.1007/s00125-024-06267-5
Hemani G, Tilling K, George Davey Smith. Orienting the causal relationship between Imprecisely measured traits using GWAS Summary Data. PLoS Genet. 2017;13(11):e1007081.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. https://doi.org/10.7554/eLife.34408 . PMID: 29846171; PMCID: PMC5976434.
Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, Joyce Y, Tung DA, Hinds RH, Perlis, and Ashley R. Winslow. Identification of 15 genetic loci Associated with risk of Major Depression in individuals of European descent. Nat Genet. 2016;48(9):1031–36.
Iwata K, Matsuzaki H, Tachibana T, Ohno K, Yoshimura S, Takamura H, Yamada K, et al. N-Ethylmaleimide-sensitive factor interacts with the Serotonin Transporter and modulates its trafficking: implications for pathophysiology in Autism. Mol Autism. 2014;5:33.
Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol. 2001;63(2):125–49.
Jansen AG, Gwen C, Dieleman PR, Jansen FC, Verhulst D, Posthuma, Tinca JC, Polderman. Psychiatric Polygenic Risk scores as Predictor for attention Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in a clinical child and adolescent sample. Behav Genet. 2020;50(4):203–12.
Kerimov N, Hayhurst JD, Peikova K, Manning JR, Walter P, Kolberg L, Samoviča M et al. 2021. eQTL Catalogue: A Compendium of Uniformly Processed Human Gene Expression and Splicing QTLs. bioRxiv. https://doi.org/10.1101/2020.01.29.924266
Kern JK, Geier DA, Sykes LK, Geier MR, Deth RC. Are ASD and ADHD a Continuum? A comparison of pathophysiological similarities between the disorders. J Atten Disord. 2015;19(9):805–27.
Khachadourian V, Mahjani B, Sandin S, Kolevzon A, Buxbaum JD. Abraham Reichenberg, and Magdalena Janecka. 2023. Comorbidities in Autism Spectrum Disorder and their etiologies. Translational Psychiatry 13 (1): 71.
Kuleshov MV, Jones MR, Rouillard AD, Nicolas F, Fernandez Q, Duan Z, Wang S Koplev, et al. Enrichr: a Comprehensive Gene Set Enrichment Analysis web server 2016 Update. Nucleic Acids Res. 2016;44(W1):W90–97.
Kwong A, Boughton AP, Wang M, VandeHaar P, Boehnke M. Gonçalo Abecasis, and Hyun Min Kang. 2022. FIVEx: an interactive eQTL browser across Public Datasets. Bioinformatics 38 (2): 559–61.
Lake BB, Chen S, Sos BC, et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat Biotechnol. 2018;36(1):70–80. https://doi.org/10.1038/nbt.4038 .
Li H. 2013. Aligning sequence reads, clone sequences and Assembly contigs with BWA-MEM. http://arxiv.org/abs/1303.3997
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, R. and 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map Format and SAMtools. Bioinformatics. 2009;25(16):2078.
Lin J, Tabassum R, Ripatti S, Pirinen M. MetaPhat: detecting and decomposing Multivariate associations from Univariate Genome-Wide Association Statistics. Front Genet. 2020;11:431.
Lin J, Mars N, Fu Y, Ripatti P, Kiiskinen T, Tukiainen T, Ripatti S. and Matti Pirinen. Integration of Biomarker Polygenic Risk Score Improves Prediction of Coronary Heart Disease. JACC: Basic to Translational Science. 2023. https://doi.org/10.1016/j.jacbts.2023.07.006
Lutz SM, Voorhies K, Wu AC, Hokanson J, Vansteelandt S, and Christoph Lange. The influence of unmeasured confounding on the MR Steiger Approach. Genet Epidemiol. 2022;46(2):139–41.
MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, Junkins H, et al. The New NHGRI-EBI Catalog of Published Genome-Wide Association Studies (GWAS catalog). Nucleic Acids Res. 2017;45(D1):D896–901.
Mattheisen M, Grove J, Als TD, Martin J, Voloudakis G, Meier S, Demontis D, et al. Identification of Shared and differentiating Genetic Architecture for Autism Spectrum Disorder, attention-deficit hyperactivity disorder and case subgroups. Nat Genet. 2022a;54(10):1470–78.
Mattheisen M, Grove J, Als TD, Martin J, Voloudakis G, Meier S, Demontis D, Bendl J, Walters R, Carey CE, Rosengren A, Strom NI, Hauberg ME, Zeng B, Hoffman G, Zhang W, Bybjerg-Grauholm J, Bækvad-Hansen M, Agerbo E, Cormand B, Nordentoft M, Werge T, Mors O, Hougaard DM, Buxbaum JD, Faraone SV, Franke B, Dalsgaard S, Mortensen PB, Robinson EB, Roussos P, Neale BM, Daly MJ, Børglum AD. Identification of shared and differentiating genetic architecture for autism spectrum disorder, attention-deficit hyperactivity disorder and case subgroups. Nat Genet. 2022b;54(10):1470–8. https://doi.org/10.1038/s41588-022-01171-3 . Epub 2022 Sep 26. PMID: 36163277; PMCID: PMC10848300.
Mazitov T, Bregin A, Philips M-A, Innos Jürgen, and Eero Vasar. Deficit in emotional learning in Neurotrimin Knockout Mice. Behav Brain Res. 2017;317(January):311–18.
McLaren W, Gil L, Hunt SE, Riat HS, Graham RS, Ritchie. Anja Thormann, Paul Flicek, and Fiona Cunningham. 2016. The Ensembl variant effect predictor. Genome Biol 17 (1): 1–14.
Morton JT, Dong-Min Jin RH, Mills Y, Shao G, Rahman D, McDonald Q, Zhu, et al. Multi-level analysis of the gut-brain Axis shows Autism Spectrum Disorder-Associated Molecular and Microbial profiles. Nat Neurosci. 2023;26(7):1208–17.
Nayar K, Sealock JM, Maltman N, Bush L, Cook EH, Lea K, Davis, and Molly Losh. Elevated polygenic burden for Autism Spectrum disorder is Associated with the broad autism phenotype in mothers of individuals with Autism Spectrum Disorder. Biol Psychiatry. 2021;89(5):476–85.
Nesterova AP, Yuryev A, Klimov EA, Zharkova M, Shkrob M, Ivanikova NV. Sergey Sozin, and Vladimir Sobolev. 2019. Disease pathways: an Atlas of Human Disease Signaling pathways . Elsevier.
Okbay A, Wu Y, Wang N, Jayashankar H, Bennett M, Nehzati SM, Sidorenko J, et al. Polygenic prediction of Educational Attainment within and between families from Genome-Wide Association Analyses in 3 million individuals. Nat Genet. 2022;54(4):437–49.
Palmer TM, Debbie A, Lawlor RM, Harbord NA, Sheehan JH, Tobias, Nicholas J, Timpson GD, Smith, Jonathan AC, Sterne. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223–42.
Pedersen CB, Bybjerg-Grauholm J, Pedersen MG, Grove J, Agerbo E, Bækvad-Hansen M, Poulsen JB, et al. The iPSYCH2012 case-cohort sample: new directions for unravelling genetic and Environmental Architectures of severe Mental disorders. Mol Psychiatry. 2018;23(1):6–14.
Peyre H, Schoeler T, Liu C, Michèle C, Williams N, Hoertel A, Havdahl, Jean-Baptiste P. Combining Multivariate genomic approaches to elucidate the comorbidity between Autism Spectrum disorder and attention deficit hyperactivity disorder. J Child Psychol Psychiatry Allied Discip. 2021;62(11):1285–96.
Phillips AN, Smith GD. How independent are ‘Independent’ effects? Relative risk estimation when correlated exposures are measured imprecisely. J Clin Epidemiol. 1991;44(11):1223–31.
Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, Thiruvahindrapuram B, et al. Convergence of genes and Cellular pathways Dysregulated in Autism Spectrum disorders. Am J Hum Genet. 2014;94(5):677–94.
Piovesan A, Caracausi M, Antonaros F, Pelleri MC, Vitale L. GeneBase 1.1: A Tool to Summarize Data from NCBI Gene Datasets and Its Application to an Update of Human Gene Statistics. Database: The Journal of Biological Databases and Curation 2016. 2016. https://doi.org/10.1093/database/baw153
Poplin R, Chang P-C, Alexander D, Schwartz S, Colthurst T, Ku A, Newburger D, et al. A Universal SNP and small-indel variant caller using deep neural networks. Nat Biotechnol. 2018;36(10):983–87.
Rees JMB, Angela M, Wood, and Stephen Burgess. Extending the MR-Egger Method for Multivariable mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med. 2017;36(29):4705–18.
Risch N, Merikangas K. The future of genetic studies of Complex Human diseases. Science. 1996;273(5281):1516–17.
Romero M, Aguilar JM, Del-Rey-Mejías Ángel, Mayoral Fermín, Rapado Marta, Peciña Marta, Barbancho Miguel Ángel, Ruiz-Veguilla Miguel, Lara José Pablo. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16(3):266–75.
Ruotsalainen SE, Juulia J, Partanen A, Cichonska J, Lin C, Benner I, Surakka FG, et al. An expanded analysis Framework for Multivariate GWAS connects inflammatory biomarkers to functional variants and Disease. Eur J Hum Genetics: EJHG. 2021;29(2):309–24.
Sadik A, Dardani C, Pagoni P, Havdahl A, Stergiakouli E, iPSYCH Autism Spectrum Disorder Working Group, Khandaker GM, et al. Parental inflammatory bowel Disease and Autism in Children. Nat Med. 2022;28(7):1406–11.
Safran M, Dalah I, Alexander J, Rosen N, Stein TI, Shmoish M, et al. GeneCards Version 3: the human gene integrator. Database. 2010;2010:baq020.
Schaaf CP, Betancur C, Yuen RKC, Jeremy R, Parr DH, Skuse L, Gallagher RA, Bernier, et al. A Framework for an evidence-based gene list relevant to Autism Spectrum Disorder. Nat Rev Genet. 2020;21(6):367–76.
Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–64.
Singh K, Jayaram M, Kaare M, Leidmaa E, Jagomäe T, Heinla I, Hickey MA, et al. Neural cell adhesion Molecule Negr1 Deficiency in Mouse results in structural brain endophenotypes and behavioral deviations related to Psychiatric disorders. Sci Rep. 2019;9(1):5457.
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of Observational studies in Epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233.
Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen Sven Cichon, et al. Psychiatric genomics: an update and an agenda. Am J Psychiatry. 2018;175(1):15–27.
Suzuki H, Yoshida T, Morisada N, Uehara T, Kosaki K, Sato K, Matsubara K, Takano-Shimizu T, and Toshiki Takenouchi. De Novo NSF mutations cause early infantile epileptic Encephalopathy. Ann Clin Transl Neurol. 2019;6(11):2334–39.
Tamlander M, Mars N, Pirinen M, FinnGen E, Widén, and Samuli Ripatti. Integration of Questionnaire-based risk factors improves polygenic risk scores for human coronary heart disease and type 2 diabetes. Commun Biology. 2022;5(1):158.
Article CAS Google Scholar
Tamm L, Duncan A, Vaughn A, McDade R, Estell N, Birnschein A, and Lori Crosby. Academic needs in Middle School: perspectives of parents and youth with autism. J Autism Dev Disord. 2020;50(9):3126–39.
Tesi N, van der Lee S, Hulsman M, Holstege H, Marcel JT, Reinders. snpXplorer: a web application to explore human SNP-Associations and annotate SNP-Sets. Nucleic Acids Res. 2021;49(W1):W603–12.
Troisi J, Autio R, Beopoulos T, Bravaccio C, Carraturo F, Corrivetti G, Cunningham S, et al. Genome, Environment, Microbiome and Metabolome in Autism (GEMMA) Study Design: biomarkers identification for Precision Treatment and Primary Prevention of Autism Spectrum disorders by an Integrated Multi-omics Systems Biology Approach. Brain Sci. 2020;10(10). https://doi.org/10.3390/brainsci10100743 .
Turley P, Walters RK, Maghzian O, Okbay A, Mark Lee JJ, Fontana Alan, Nguyen-Viet Tuan Anh, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50(2):229–37.
Van den Hove DLA, Kenis G, Brass A, Opstelten R, Rutten BPF, Bruschettini M, Blanco CE, Lesch KP, Steinbusch HWM, Prickaerts J. Vulnerability versus resilience to prenatal stress in male and female rats; implications from Gene expression profiles in the Hippocampus and Frontal Cortex. Eur Neuropsychopharmacology: J Eur Coll Neuropsychopharmacol. 2013;23(10):1226–46.
Vgontzas A, and William Renthal. Migraine-Associated Gene expression in cell types of the Central and Peripheral Nervous System. Cephalalgia: Int J Headache. 2020;40(5):517–23.
Wallace C. Eliciting priors and relaxing the single causal variant Assumption in Colocalisation analyses. PLoS Genet. 2020;16(4):e1008720.
Wallace C. A more Accurate Method for Colocalisation Analysis allowing for multiple causal variants. PLoS Genet. 2021;17(9):e1009440.
Xie M-J, Iwata K, Ishikawa Y, Nomura Y, Tani T, Murata K, Fukazawa Y, and Hideo Matsuzaki. Autistic-like Behavior and Impairment of Serotonin Transporter and AMPA receptor trafficking in N-Ethylmaleimide sensitive factor gene-deficient mice. Front Genet. 2021;12:748627.
Download references
Acknowledgements
The work was supported by the European Commission Horizon 2020 programme (grant no. 825033) and the Strategic profiling of Tampere University in health data science, Academy of Finland, PROFI 6, 2021-2026. We are sincerely grateful to the entire GEMMA team and in particular to the nurses, recruiters and especially families participating in the project.
Open access funding provided by Tampere University (including Tampere University Hospital). The work was supported by the GEMMA project, funded by the European Commission by means of the Horizon 2020 program (call H2020-SC1-BHC-03-2018) with the project ID 825033 and the Strategic profiling of Tampere University in health data science, Academy of Finland, PROFI 6, 2021–2026.
Author information
Karoliina Salenius, Niina Väljä, Reija Autio and Jake Lin contributed equally to this work.
Authors and Affiliations
Faculty of Medicine and Health Technology, Tampere University and Tays Cancer Centre, Tampere, Finland
Karoliina Salenius, Niina Väljä, Sini Thusberg, Matti Nykter & Jake Lin
BMSystems, Paris, France
Francois Iris
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, USA
Christine Ladd-Acosta
Euformatics, Tekniikantie, Espoo, Finland
Christophe Roos
Foundation for the Finnish Cancer Institute, Helsinki, Finland
Matti Nykter
European Biomedical Research Institute of Salerno (EBRIS), Salerno, Italy
Alessio Fasano
Harvard Medical School, Harvard T.H. Chan School of Public Health, Boston, USA
Health Sciences, Faculty of Social Sciences, Tampere University, Tampere, Finland
Reija Autio
Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden
You can also search for this author in PubMed Google Scholar
Contributions
JL, KS, CLA, CR, MN and RA designed the study. NV, KS, ST, RA and JL performed the data analysis and integration. KS, NV, ST, FI, RA and JL conducted the validation and figure generation. KS, FI, NV, AF, CR, RA and JL wrote the paper. All authors contributed to critical revisions and approved the final manuscript.
Corresponding author
Correspondence to Jake Lin .
Ethics declarations
Ethics approval and consent to participate.
The GEMMA study was approved by the relevant ethics committee of each enrolling country. Particularly, CE Campania Sud (IRB n.30/2019) for Italy; Partners Human Research (IRB ver.01/04/2019) for USA; and Clinical Research Ethics Committee of Galway University Hospital (IRB n. C.A. 2127/19) for Ireland. A written consent form will be signed by each participant or their legal representative.
Consent for publication
Consent, relevant to GEMMA subjects, is granted and signed by each participant or their legal representative.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., 12888_2024_6392_moesm3_esm.pdf.
Supplementary Figure 1. Genetic correlation of the traits included in the analysis. ASD = Autism Spectrum Disorder; ADHD = Attention Deficit Hyper Disorder; ASRD = Anxiety-Stress Disorder; DBD = Disruptive Behaviour Disorder; EA = Education attainment; MDD = Major Depression Disorder; SCZ = Schizophrenia. Supplementary Figure 2 . P -value and BIC decomposition processing of MAPT and NSF to identify ASD central traits. ASD=Autism Spectrum Disorder; ADHD=Attention Deficit Hyper Disorder; ASRD=Anxiety-Stress Disorder; DBD=Disruptive Behaviour Disorder; EA=Education attainment; MDD=Major Depression Disorder; SCZ=Schizophrenia. Supplementary Figure 3. Pathway analysis using the WikiPathway database also highlights neuronal processes, with bar length and color indicating significance. More details listed in Supplementary Table 8. Supplementary Figure 4. Tissue and cell (TS) type enrichment using WebCSEA and the list of the 22 central trait genes found that the most enriched tissues are related to cerebrum, cortex and small intestine related tissue types. Lake 2017 refers to data from human brain single cell analysis project (https://pubmed.ncbi.nlm.nih.gov/29227469/) while HCA stands for histologic chorioamnionitis, an intrauterine inflammatory trait. Supplementary Figure 5. ASD multivariate GWAS associations within the MAPT H1/H2 haplotype, 17q21 arm region, are presented in a Manhattan plot, in the context of Grove et al. GWAS results. Significance thresholds for p -values of 1e-05 indicated in blue and 1e-08 in red. Significant SNPs highlighted in green show rs62061734 (MAPT), rs269633 (KANSL1) and rs538628 (NSF).
12888_2024_6392_MOESM4_ESM.xlsx
Supplementary Table 1. Data and sample details of ASD and 8 genetically correlated traits ( P < 0.05, calculated from LD Score Regression (LDSC)) are presented and applied towards multivariate-GWAS. Data from four excluded traits are additionally shown. Supplementary Table 2. 37 multivariate associations are identified with ASD as a central trait where 17/37, shown with asterisk are previously reported in the GWAS Catalog and in bold, 8 genes are identified as SFARI ASD genes. Supplementary Table 3. 19 gene regions/trait pairings passed coloc (Posterior Prob H4 > 90%, Shown in bold) called on coloc.abf with a window size of ± 50 KB flanking the SNP locus. Supplementary Table 4. (A) Mendelian randomization (MR) results for ASD as outcome and related traits. (B) MR where ASD is the exposure and related traits are the outcome. Supplementary Table 5. MV associated genes are found in systems curated/implicated with gut microbiome and neural systems from GeneCards. Supplementary Table 6. List of 637 Significant SNPs ( p < 5e-8), with 315 already reported in the GWAS catalog, identified by MetaPhat multivariate-GWAS using ASD and 8 genetically correlated trait summary statistics. Supplementary Table 7. A) 108 enriched ( p < 0.05) Go terms are annotated and (B) 46 pathways on WikiPathway C) KEGG D) Reactome resources e) Tissue from the list of multivariate ASD SNPs found enrichments in neuron and nervous systems related data. Supplementary Table 8. ASD central SNP alleles are mapped to GEMMA genotypes called from 112 (49% females) WGS samples (45 (42% females) ASD probands). Phi coefficients are calculated between allele proportions where Chi-square test is applied to assess statistical importance. Indicated with *. Fisher's exact test is applied when Chi-square assumptions are violated. Supplementary Table 9. eQTL and sQTL related results of the ASD central associations relative to brain and nervous systems from EBI QTL catalog are captured via https://fivex.sph.umich.edu/. Study URLs are listed at the bottom of the table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Salenius, K., Väljä, N., Thusberg, S. et al. Exploring autism spectrum disorder and co-occurring trait associations to elucidate multivariate genetic mechanisms and insights. BMC Psychiatry 24 , 934 (2024). https://doi.org/10.1186/s12888-024-06392-w
Download citation
Received : 01 August 2024
Accepted : 08 December 2024
Published : 18 December 2024
DOI : https://doi.org/10.1186/s12888-024-06392-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- ASD genetically correlated traits
- Multivariate GWAS
- Mendelian randomization
BMC Psychiatry
ISSN: 1471-244X
- General enquiries: [email protected]
Genetic discovery links new gene to autism spectrum disorder
Variants in the ddx53 and other genes on the x chromosome provide genetic clues to male prevalence in asd.
New research published in The American Journal of Human Genetics has identified a previously unknown genetic link to autism spectrum disorder (ASD). The study found that variants in the DDX53 gene contribute to ASD, providing new insights into the genetic underpinnings of the condition.
ASD, which affects more males than females, encompasses a group of neurodevelopmental conditions that result in challenges related to communication, social understanding and behaviour. While DDX53 , located on the X chromosome, is known to play a role in brain development and function, it was not previously definitively associated with autism.
In the study published today, researchers from The Hospital for Sick Children (SickKids) in Canada and the Istituto Giannina Gaslini in Italy clinically tested 10 individuals with ASD from 8 different families and found that variants in the DDX53 gene were maternally inherited and present in these individuals. Notably, the majority were male, highlighting the gene's potential role in the male predominance observed in ASD.
"By pinpointing DDX53 as a key player, particularly in males, we can better understand the biological mechanisms at play and improve diagnostic accuracy for individuals and their families," says senior author Dr. Stephen Scherer, Senior Scientist, Genetics & Genome Biology and Chief of Research at SickKids, and Director of the McLaughlin Centre at the University of Toronto.
"Identifying this new gene as a confirmed contributor to ASD underscores the complexity of autism and the need for comprehensive genetic analysis."
At the same location on the X chromosome, the researchers found evidence that another gene, PTCHD1-AS , might be involved in autism. The study highlights a case where a boy and his mother, both with autism with little support needs, had a specific gene deletion involving the DDX53 gene and parts of PTCHD1-AS .
The study cohort was assembled through an international collaborative effort, involving several renowned clinical and research institutions from Canada, Italy and the U.S. Further analysis of large autism research databases, including Autism Speaks MSSNG and Simons Foundation Autism Research Initiative, identified 26 more individuals with ASD who had similar rare DDX53 variants to the study participants.
"This gene has long eluded us, not previously linked to any neuropsychiatric condition. Our findings support a direct link between DDX53 and autism, which is not only crucial for future clinical genetic testing, but its discovery suggests that the pathway it affects is related to the behavioural traits of autism, opening a whole new area of exploration," says lead author Dr. Marcello Scala, researcher in Medical Genetics at the Istituto Giannina Gaslini, affiliated with the University of Genoa (Department of Neuroscience).
In another paper published today in the same journal, Scherer and lead author Dr. Marla Mendes, a research fellow at SickKids, identified 59 genetic variants on the X chromosome significantly associated with ASD. The variants were found in genes linked to autism, including PTCHD1-AS (near to DDX53 ), DMD , HDAC8 , PCDH11X, and PCDH19 beside novel ASD-linked candidates ASB11 and ASB9 . Additionally, the FGF13 gene was highlighted as being related to ASD, with sex-specific differences, adding more evidence to the role of sex chromosomes in the condition.
"These findings provide new insights into the biology of the X chromosome in ASD, providing additional evidence for the involvement of certain genes like DDX53 and FGF13 , and suggesting they should be investigated further," says Scherer.
The team notes that the absence of a similar gene like DDX53 in commonly used mouse models may require future researchers to reconsider how they study ASD. Since it lacks a functional equivalent in these models, findings in DDX53 cannot be easily replicated.
"Insights from this study could significantly influence the design and interpretation of autism research, particularly in developing new models. Identifying these variants is an important step towards developing more precise diagnostics and therapeutics for patients and families with ASD," says Scherer.
Scherer also added "both studies provide even more evidence that complex neurobehavioral conditions like autism can sometimes have simple biologic (genetic) underpinnings."
The study was funded by the University of Toronto McLaughlin Centre, Autism Speaks, Autism Speaks Canada, Ontario Brain Institute, the Italian Ministry for Education, University and Research and SickKids Foundation. Additional funding was provided by National Institutes of Health and the California Center for Rare Diseases at UCLA.
- Birth Defects
- Gene Therapy
- Personalized Medicine
- Learning Disorders
- Disorders and Syndromes
- Child Development
- Autistic spectrum
- Bipolar disorder
- Psychiatric service dog
- Adult attention-deficit disorder
- Asperger syndrome
- Molecular biology
Story Source:
Materials provided by The Hospital for Sick Children . Note: Content may be edited for style and length.
Journal Reference :
- Marcello Scala, Clarrisa A. Bradley, Jennifer L. Howe, Brett Trost, Nelson Bautista Salazar, Carole Shum, Marla Mendes, Miriam S. Reuter, Evdokia Anagnostou, Jeffrey R. MacDonald, Sangyoon Y. Ko, Paul W. Frankland, Jessica Charlebois, Mayada Elsabbagh, Leslie Granger, George Anadiotis, Verdiana Pullano, Alfredo Brusco, Roberto Keller, Sarah Parisotto, Helio F. Pedro, Laina Lusk, Pamela Pojomovsky McDonnell, Ingo Helbig, Sureni V. Mullegama, Emilie D. Douine, Rosario Ivetth Corona, Bianca E. Russell, Stanley F. Nelson, Claudio Graziano, Maria Schwab, Laurie Simone, Federico Zara, Stephen W. Scherer. Genetic variants in DDX53 contribute to autism spectrum disorder associated with the Xp22.11 locus . The American Journal of Human Genetics , 2024; DOI: 10.1016/j.ajhg.2024.11.003
Cite This Page :
Explore More
- Can the Heart Heal Itself? New Study Says It Can
- Tinkering With 'Clockwork' Mechanisms of Life
- Quantum Teleportation Over Busy Internet Cables
- Mysteries of Icy Ocean Worlds
- Safer Spuds: Removing Toxins from Potatoes
- Gruel Eaten by Early Neolithic Farmers
- Dark Energy 'Doesn't Exist'
- Nerve Regeneration After Spinal Cord Injury
- Laser-Based Artificial Neuron: Lightning Speed
- Large Hadron Collider Regularly Makes Magic
Trending Topics
Strange & offbeat.

New Research May Change How We Think About the Autism Spectrum
Insar keynote suggests brain differences correlate with cognition—not diagnosis..
Posted May 16, 2022 | Reviewed by Davia Sills
- What Is Autism?
- Find a therapist to help with autism
- Dr. Evdokia Anagnostou presented the results of neuroimaging studies at the International Society for Autism Research 2022 annual meeting.
- Of note, brain differences clustered along dimensions of cognition and hyperactivity, not diagnosis.
- These findings suggest we need to reconsider how we classify neurodivergence.
University of Toronto child neurologist Evdokia Anagnostou dropped a bombshell in her keynote Saturday at the annual meeting of the International Society of Autism Research (INSAR) in Austin, Texas, which may call into question the validity of the autism spectrum disorder (ASD) diagnosis.
What Brain Scans Tell Us About Autism Spectrum Disorder
Anagnostou and her colleagues had set out to use neuroimaging to identify brain differences unique to ASD, as compared to other neurodevelopmental differences like ADHD , OCD , and intellectual disability. And they did find that brain differences clustered into different groups—but not by diagnosis. In fact, brain scans could not distinguish children who had been diagnosed with ASD from those who had been diagnosed with ADHD or OCD.
“Dr. Anagnostou reported data from multiple papers that looked at over 3,500 children,” Dr. Alycia Halladay, Chief Science Officer at the Autism Science Foundation, explained to me. “These studies looked at multiple structural and functional features of the brain—including cortical gyrification (the way the brain folds in the cortex), connectivity of different brain regions, and the thickness of the cortical area—and found no differences based on diagnosis.”
Groupings did emerge, but they were along totally different axes. Added Halladay, “The brains themselves were more similar based on cognitive ability, hyperactivity, and adaptive behavior.” In other words, the brains of mildly affected autistic children looked much more like the brains of kids with ADHD than they did like those of severely autistic children.
Validity of the Autism Spectrum Diagnosis May Be at Stake
If replicated, these findings could have tremendous implications for our current diagnostic framework. During the question and answer period following her talk, Anagnostou described two children who both carried the diagnosis of autism; one was very mildly affected, while the other had such disordered behavior that “even their bus driver knows” he is autistic. “Should these kids have the same diagnosis?” she asked.
Right now, they do—but there has been a growing dissatisfaction among many stakeholders in the autism community with the American Psychiatric Association’s introduction of the all-encompassing ASD diagnosis in the 2013 revision of the Diagnostic and Statistical Manual (DSM-5) to replace more narrowly defined categories, including Asperger syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS), and childhood disintegrative disorder.
In 2021, the Lancet Commission —a group of 32 researchers, clinicians, autistic individuals, and family members—called for the creation of a new label, “profound autism,” that would carve out those autistic individuals who also suffer from cognitive and language impairments and require round-the-clock supervision. “Anagnostou’s data converge nicely with the Lancet Commission’s proposal,” Halladay observed. “They provide biological evidence for a category that was originally defined solely by external criteria.”
At the very least. The real question is whether this work demands an even more radical re-imagining of our classification of neurodevelopmental differences. If, as Anagnostou’s data demonstrates, cognition and hyperactivity are much more correlated with brain difference than variables like social deficit that have been considered core symptoms of autism, then perhaps it’s time to scrap our current model and introduce new diagnoses based on these more salient dimensions. Aligning our diagnostic system with underlying biology is the first step in the development of targeted interventions for some of the most intractable and dangerous behaviors exhibited by the developmentally disabled, such as aggression , elopement, self-injury , and pica (the compulsion to eat inedible objects).
As Anagnostou opened her talk, “Nature doesn’t read the DSM.” But, as our understanding of the brain advances, shouldn’t the DSM reflect these divisions in nature?

Amy S.F. Lutz, Ph.D. , is a historian of medicine at the University of Pennsylvania. She is the author of We Walk: Life with Severe Autism (2020) and Each Day I Like It Better: Autism, ECT, and the Treatment of Our Most Impaired Children (2014) . She is also the Vice-President of the National Council on Severe Autism (NCSA).
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

When we fall prey to perfectionism, we think we’re honorably aspiring to be our very best, but often we’re really just setting ourselves up for failure, as perfection is impossible and its pursuit inevitably backfires.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience

IMAGES
COMMENTS
The high prevalence and high rank for non-fatal health burden of autism spectrum disorder in people younger than 20 years underscore the importance of early detection and support to autistic young people and their caregivers globally. Work to improve the precision and global representation of our findings is required, starting with better global coverage of epidemiological data so that ...
From studies of the connections between neurons to interactions between the nervous and immune systems to the complex ways in which people understand not just language, but also the unspoken nuances of conversation, new research projects at MIT supported by the Simons Center for the Social Brain are bringing a rich diversity of perspectives to advancing the field's understanding of autism.
From studies of the connections between neurons to interactions between the nervous and immune systems to the complex ways in which people understand not just language, but also the unspoken nuances of conversation, new research projects at MIT supported by the Simons Center for the Social Brain are bringing a rich diversity of perspectives to advancing the field's understanding of autism.
New research shows that providing support is beneficial for both the parents and the child outcome, and elevates strengths while mitigating support challenges. ... Autism Research 2022; n/a(n/a). 71. Pham HH, Sandberg N, Trinkl J, Thayer J. Racial and Ethnic Differences in Rates and Age of Diagnosis of Autism Spectrum Disorder.
Background Autism spectrum disorder (ASD) is a partially heritable neurodevelopmental trait, and people with ASD may also have other co-occurring trait such as ADHD, anxiety disorders, depression, mental health issues, learning difficulty, physical health traits and communication challenges. The concomitant development of ASD and other neurological traits is assumed to result from a complex ...
Overview. The 2022 IACC Summary of Advances in Autism Research summaries of the top 20 advances in autism biomedical and services research selected by members of the Interagency Autism Coordinating Committee (IACC). Articles in the Summary of Advances are grouped according to the topics represented by the seven Questions of the 2021-2023 IACC Strategic Plan for Autism Research, Services, and ...
New research published in The American Journal of Human Genetics has identified a previously unknown genetic link to autism spectrum disorder (ASD). The study found that variants in the DDX53 gene ...
The research was carried out on autism animal models. The researchers discovered changes in the brain, including a decrease in neuroinflammation, which has been linked to autism. ... Gilad Levy, Ela Bar, Sari Schokoroy Trangle, Shai Efrati and Boaz Barak, 21 September 2022, International Journal of Molecular ... I don't think that the chamber ...
The 2022 Summary of Advances meets the requirements of the Autism Collaboration, Accountability, Research, Education, and Support (Autism CARES) Act of 2019. *** The IACC is a federal advisory committee that was created by Congress in an effort to accelerate progress in autism research and services.
Dr. Evdokia Anagnostou presented the results of neuroimaging studies at the International Society for Autism Research 2022 annual meeting. Of note, brain differences clustered along dimensions of ...