
- Why Does Water Expand When It Freezes
Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks

Who did the Gold Foil Experiment?
The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom . Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of Manchester between 1908 and 1913.
The prevalent atomic theory at the time of the research was the plum pudding model that was developed by Lord Kelvin and further improved by J.J. Thomson. According to the theory, an atom was a positively charged sphere with the electrons embedded in it like plums in a Christmas pudding.
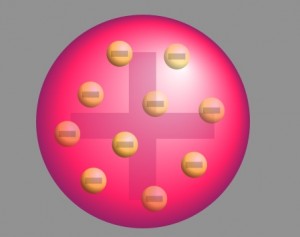
With neutrons and protons yet to be discovered, the theory was derived following the classical Newtonian Physics. However, in the absence of experimental proof, this approach lacked proper acceptance by the scientific community.
What is the Gold Foil Experiment?
Description.
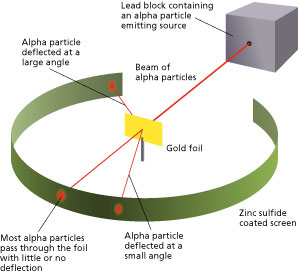
The method used by scientists included the following experimental steps and procedure. They bombarded a thin gold foil of thickness approximately 8.6 x 10 -6 cm with a beam of alpha particles in a vacuum. Alpha particles are positively charged particles with a mass of about four times that of a hydrogen atom and are found in radioactive natural substances. They used gold since it is highly malleable, producing sheets that can be only a few atoms thick, thereby ensuring smooth passage of the alpha particles. A circular screen coated with zinc sulfide surrounded the foil. Since the positively charged alpha particles possess mass and move very fast, it was hypothesized that they would penetrate the thin gold foil and land themselves on the screen, producing fluorescence in the part they struck.
Like the plum pudding model, since the positive charge of atoms was evenly distributed and too small as compared to that of the alpha particles, the deflection of the particulate matter was predicted to be less than a small fraction of a degree.
Observation
Though most of the alpha particles behaved as expected, there was a noticeable fraction of particles that got scattered by angles greater than 90 degrees. There were about 1 in every 2000 particles that got scattered by a full 180 degree, i.e., they retraced their path after hitting the gold foil.
Simulation of Rutherford’s Gold Foil Experiment Courtesy: University of Colorado Boulder
The unexpected outcome could have only one explanation – a highly concentrated positive charge at the center of an atom that caused an electrostatic repulsion of the particles strong enough to bounce them back to their source. The particles that got deflected by huge angles passed close to the said concentrated mass. Most of the particles moved undeviated as there was no obstruction to their path, proving that the majority of an atom is empty.
In addition to the above, Rutherford concluded that since the central core could deflect the dense alpha particles, it shows that almost the entire mass of the atom is concentrated there. Rutherford named it the “nucleus” after experimenting with various gases. He also used materials other than gold for the foil, though the gold foil version gained the most popularity.
He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. In this model, a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. As a result of his gold foil experiment, Rutherford’s atomic theory holds good even today.
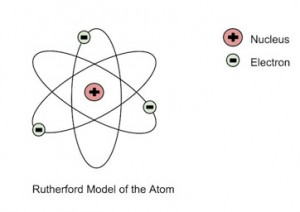
Rutherford’s Atomic Model
Rutherford’s Gold Foil Experiment Animation
- Rutherford demonstrated his experiment on bombarding thin gold foil with alpha particles contributed immensely to the atomic theory by proposing his nuclear atomic model.
- The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
- The significance and purpose of the gold foil experiment are still prevalent today. The discovery of the nucleus paved the way for further research, unraveling a list of unknown fundamental particles.
- Chemed.chem.purdue.edu
- Chem.libretexts.org
- Large.stanford.edu
- Radioa ctivity.eu.com
Article was last reviewed on Friday, February 3, 2023
Related articles

5 responses to “Gold Foil Experiment”
Super very much helpful to me,clear explanation about every act done by our Rutherford that is under different sub headings ,which is very much clear to ,to study .very much thanks to the science facts.com.thank u so much.
Good explanation,very helpful ,thank u ,so much
very clear and helpful, perfect for my science project!
Thank you for sharing the interactive program on the effects of the type of atom on the experiment! Looking forward to sharing this with my ninth graders!
Rutherford spearheaded with a team of scientist in his experiment of gold foil to capture the particles of the year 1911. It’s the beginning of explaining particles that float and are compacted . Rutherford discovered this atom through countless experiments which was the revolutionary discovery of the atomic nuclear . Rutherford name the atom as a positive charge and the the center is the nucleus.
Barack Hussein Obama
Mrs. Danize Obama
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
Experimental Evidence for the Structure of the Atom
George sivulka march 23, 2017, submitted as coursework for ph241 , stanford university, winter 2017, introduction.
The Rutherford Gold Foil Experiment offered the first experimental evidence that led to the discovery of the nucleus of the atom as a small, dense, and positively charged atomic core. Also known as the Geiger-Marsden Experiments, the discovery actually involved a series of experiments performed by Hans Geiger and Ernest Marsden under Ernest Rutherford. With Geiger and Marsden's experimental evidence, Rutherford deduced a model of the atom, discovering the atomic nucleus. His "Rutherford Model", outlining a tiny positively charged atomic center surrounded by orbiting electrons, was a pivotal scientific discovery revealing the structure of the atoms that comprise all the matter in the universe.
The experimental evidence behind the discovery involved the scattering of a particle beam after passing through a thin gold foil obstruction. The particles used for the experiment - alpha particles - are positive, dense, and can be emitted by a radioactive source. Ernest Rutherford discovered the alpha particle as a positive radioactive emission in 1899, and deduced its charge and mass properties in 1913 by analyzing the charge it induced in the air around it. [1] As these alpha particles have a significant positive charge, any significant potential interference would have to be caused by a large concentration of electrostatic force somewhere in the structure of the atom. [2]
Previous Model of the Atom
The scattering of an alpha particle beam should have been impossible according to the accepted model of the atom at the time. This model, outlined by Lord Kelvin and expanded upon by J. J. Thompson following his discovery of the electron, held that atoms were comprised of a sphere of positive electric charge dotted by the presence of negatively charged electrons. [3] Describing an atomic model similar to "plum pudding," it was assumed that electrons were distributed throughout this positive charge field, like plums distributed in the dessert. However, this plum pudding model lacked the presence of any significant concentration of electromagnetic force that could tangibly affect any alpha particles passing through atoms. As such, alpha particles should show no signs of scattering when passing through thin matter. [4] (see Fig. 2)
The Geiger Marsden Experiments
Testing this accepted theory, Hans Geiger and Ernest Marsden discovered that atoms indeed scattered alpha particles, a experimental result completely contrary to Thompson's model of the atom. In 1908, the first paper of the series of experiments was published, outlining the apparatus used to determine this scattering and the scattering results at small angles. Geiger constructed a two meter long glass tube, capped off on one end by radium source of alpha particles and on the other end by a phosphorescent screen that emitted light when hit by a particle. (see Fig. 3) Alpha particles traveled down the length of the tube, through a slit in the middle and hit the screen detector, producing scintillations of light that marked their point of incidence. Geiger noted that "in a good vacuum, hardly and scintillations were observed outside of the geometric image of the slit, "while when the slit was covered by gold leaf, the area of the observed scintillations was much broader and "the difference in distribution could be noted with the naked eye." [5]
On Rutherford's request, Geiger and Marsden continued to test for scattering at larger angles and under different experimental parameters, collecting the data that enabled Rutherford to further his own conclusions about the nature of the nucleus. By 1909, Geiger and Marsden showed the reflection of alpha particles at angles greater than 90 degrees by angling the alpha particle source towards a foil sheet reflector that then would theoretically reflect incident particles at the detection screen. Separating the particle source and the detector screen by a lead barrier to reduce stray emission, they noted that 1 in every 8000 alpha particles indeed reflected at the obtuse angles required by the reflection of metal sheet and onto the screen on the other side. [6] Moreover, in 1910, Geiger improved the design of his first vacuum tube experiment, making it easier to measure deflection distance, vary foil types and thicknesses, and adjust the alpha particle stream' velocity with mica and aluminum obstructions. Here he discovered that both thicker foil and foils made of elements of increased atomic weight resulted in an increased most probable scattering angle. Additionally, he confirmed that the probability for an angle of reflection greater than 90 degrees was "vanishingly small" and noted that increased particle velocity decreased the most probably scattering angle. [7]

Rutherford's Atom
Backed by this experimental evidence, Rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; as large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. As he concludes this reasoning with the "simplest explanation" in his 1911 paper, the "atom contains a central charge distributed through a very small volume" and "the large single deflexions are due to the central charge as a whole." In fact, he mathematically modeled the scattering patterns predicted by this model with this small central "nucleus" to be a point charge. Geiger and Marsden later experimentally verified each of the relationships predicted in Rutherford's mathematical model with techniques and scattering apparatuses that improved upon their prior work, confirming Rutherford's atomic structure. [4, 8, 9] (see Fig. 1)
With the experimentally analyzed nature of deflection of alpha rays by thin gold foil, the truth outlining the structure of the atom falls into place. Though later slightly corrected by Quantum Mechanics effects, the understanding of the structure of the the atom today almost entirely follows form Rutherford's conclusions on the Geiger and Marsden experiments. This landmark discovery fundamentally furthered all fields of science, forever changing mankind's understanding of the world around us.
© George Sivulka. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] E. Rutherford, "Uranium Radiation and the Electrical Conduction Produced By It," Philos. Mag. 47 , 109 (1899).
[2] E. Rutherford, "The Structure of the Atom," Philos. Mag. 27 , 488 (1914).
[3] J. J. Thomson, "On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a Number of Corpuscles Arranged at Equal Intervals Around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure," Philos. Mag. 7 , 237 (1904).
[4] E. Rutherford, "The Scattering of α and β Particles by Matter and the Structure of the Atom," Philos. Mag. 21 , 669 (1911).
[5] H. Geiger, "On the Scattering of the α Particles by Matter," Proc. R. Soc. A 81 , 174 (1908).
[6] H. Geiger and E. Marsden, "On a Diffuse Reflection of the α-Particles," Proc. R. Soc. A 82 , 495 (1909).
[7] H. Geiger, "The Scattering of the α Particles by Matter," Proc. R. Soc. A 83 , 492 (1910).
[8] E. Rutherford, "The Origin of α and β Rays From Radioactive Substances," Philos. Mag. 24 , 453 (1912).
[9] H. Geiger and E. Marsden, "The Laws of Deflexion of α Particles Through Large Angles," Philos. Mag. 25 , 604 (1913).
About Rutherford's Gold Foil Experiment

Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial University of Tokyo, first proposed the theory of the nucleus as it is known today. Rutherford's "gold foil experiment" led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model." This model was developed in 1904 by J.J. Thompson, the scientist who discovered the electron. This theory held that the negatively charged electrons in an atom were floating in a sea of positive charge–the electrons being akin to plums in a bowl of pudding. Although Dr. Nagaoka had published his competing theory that electrons orbit a positive nucleus, akin to the way the planet Saturn is orbited by its rings, in 1904, the plum pudding model was the prevailing theory on the structure of the atom until it was disproved by Ernest Rutherford in 1911.
The gold foil experiment was conducted under the supervision of Rutherford at the University of Manchester in 1909 by scientist Hans Geiger (whose work eventually led to the development of the Geiger counter) and undergraduate student Ernest Marsden. Rutherford, chair of the Manchester physics department at the time of the experiment, is given primary credit for the experiment, as the theories that resulted are primarily his work. Rutherford's gold foil experiment is also sometimes referred to as the Geiger-Marsden experiment.
The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. The expected result was that the positive particles would be moved just a few degrees from their path as they passed through the sea of positive charge proposed in the plum pudding model. The result, however, was that the positive particles were repelled off of the gold foil by nearly 180 degrees in a very small region of the atom, while most of the remaining particles were not deflected at all but rather passed right through the atom.
Significance
The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. Because the majority of the positive particles continued on their original path unmoved, Rutherford correctly deducted that most of the remainder of the atom was empty space. Rutherford termed his discovery "the central charge," a region later named the nucleus.
Rutherford's discovery of the nucleus and proposed atomic structure was later refined by physicist Niels Bohr in 1913. Bohr's model of the atom, also referred to as the Rutherford Bohr model, is the basic atomic model used today. Rutherford's description of the atom set the foundation for all future atomic models and the development of nuclear physics.
Cite This Article
Pestka, Jessica. "About Rutherford's Gold Foil Experiment" sciencing.com , https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/. 24 April 2017.
Pestka, Jessica. (2017, April 24). About Rutherford's Gold Foil Experiment. sciencing.com . Retrieved from https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/
Pestka, Jessica. About Rutherford's Gold Foil Experiment last modified March 24, 2022. https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/
Recommended
Rutherford Experiment and Atomic Collisions
Claimed by: Lia McSweeney (Fall 2023)
- 1.1 A Mathematical Model
- 1.2 A Computational Model
- 2.2 Middling
- 2.3 Difficult
- 3 Connectedness
- 5.1 Further Reading
- 5.2 External Links
- 6 References
The Main Idea
Rutherford's Gold Foil Experiment helped detect that there was a large positively charged mass in the center of an atom: the nucleus. The experiment was done through the use of atomic collisions. Under the instruction of Rutherford, Hans Geiger and Ernest Marsden pointed a beam of alpha particles at a thin foil of metal and measured the scattering pattern by using a fluorescent screen. The scientists noted that some alpha particles bounced in random directions. This was not originally hypothesized due to the idea that, at most the alpha particle should experience only a 90° scattering angle. This helped lead to the discovery of the nucleus and a highly compact positively charged center.
Rutherford studied the particles that uranium and its derivatives emitted and how these particles affected certain materials. Rutherford created a method to record the position of each alpha particle by circling the bombarded object with a ZnS coated sheet. This sheet would emit a flash of light when hit by an alpha particle, allowing Rutherford to accurately measure the deflection of each alpha particle. This gave Rutherford a counting mechanism for theses particles he wanted to study. Rutherford then began to study the angles that negatively charged particles deflected when they collided with a thin metal foil. This was the beginning of his most famous study: the gold foil experiment. Knowing the relative mass of these negatively charged particles and their quick speed, he hypothesized that they would pierce the metal foil but then collide with the atoms dispersed inside the foil resulting in the small deflections. These deflections were extremely small, usually by a degree. In 1911, Ernest Rutherford took this experiment further and worked with his assistants, Hans Geiger and Ernest Marsden, to carry out an experiment that tested the plum pudding model. They shot alpha (helium 2+) particles at gold foil in order to measure the deflection of the particles as they come off of the other side. They decided to see if these deflections could occur at larger angles greater than 90 degrees. Through countless trials, they found an extremely small portion of these deflections to occur at angles larger than 90 degrees. Rutherford wondered how these large deflections occurred and concluded that there existed an extremely small and positively charged area in the atom that resulted in these huge deflections. He eventually named this area the nucleus. What happened during these deflections was that most particles would become slightly deflected by small angles due to the positive atoms. However, some would collide directly with nucleus resulting in the deflections that were greater than 90 degrees. These occurred rarely because the nucleus was such a small size so the probability of these atoms hitting the nucleus was very low. This experiment helped indicate that the atom is made predominantly of empty space with a small nucleus with protons and electrons placed extremely far away from the nucleus in their own cloud. Rutherford devised the name “proton” to describe the positive particles in the nucleus. He thought that a neutral particle existed in the nucleus too, but its existence wasn’t confirmed until 1932 when James Chadwick proved it.
A Mathematical Model
Rutherford modeled the effect the alpha particle has on the electrons of the gold atom. He did this by calculating the potential electric energy between the particle and the atom using the formula below. Rutherford came up with several equations to numerically describe these deflections. Based on the equations below, the number of particles scattered at a certain angle is directly proportional to the thickness of the metal foil and the square of the nucleus’ charge but inversely proportional to the particle’s velocity raised to the fourth power.
[math]\displaystyle{ {U_{elec}} = {\frac{1}{4πε_0}}{\frac{q_{α}q_{Au}}{r}} }[/math]
r = center to center distance between particle and atom
[math]\displaystyle{ {\frac{1}{4πε_0}} = {9*10^9}{\frac {N*m^2}{C^2}} }[/math]
[math]\displaystyle{ {q_α} }[/math] = charge of alpha particle
[math]\displaystyle{ {q_{Au}} }[/math] = charge of gold nucleus
In this instance the charge of the alpha particle is equal to 2e and the charge of the gold particle is equal to 79e.
Another important part of atomic collisions is that they are inelastic collisions. This is shown by the conservation of both momentum and kinetic energy. Take the alpha particle and gold particle for example.
[math]\displaystyle{ {\vec{p_{α,i}}} = {\vec{p_{α,f}}}+ {\vec{p_{Au,f}}} }[/math]
[math]\displaystyle{ {\vec{K_{α,i}}} = {\vec{K_{α,f}}}+ {\vec{K_{Au,f}}} }[/math]
Where [math]\displaystyle{ {\vec{p}} }[/math] is momentum and [math]\displaystyle{ {\vec{K}} }[/math] is kinetic energy.
A Computational Model
Much like the mathematical model, the collision can be modeled computationally using the same formulas. Here is a video of a VPython mode of a continuous stream of alpha particles with exaggerated interaction for easy viewing:
Example Problems
The scattering of alpha particles from nuclei is mathematically modeled from the Coulomb force and treated as an orbit. For a ZnS detector at a specific angle with respect to the incident beam, the number of particles per unit area striking the detector is given by the Rutherford formula: [math]\displaystyle{ N(θ) = {\frac{N_inLZ^2k^2e^4}{4r^2KE^2sin^4(θ/2)}} }[/math] where [math]\displaystyle{ N_i = \text {number of incident alpha particles} }[/math] [math]\displaystyle{ n = \text {atoms per unit volume in target} }[/math] [math]\displaystyle{ L = \text {thickness of target} }[/math] [math]\displaystyle{ Z = \text {atomic number of target} }[/math] [math]\displaystyle{ e = \text {electron charge} }[/math] [math]\displaystyle{ k = \text {Coulomb's constant} }[/math] [math]\displaystyle{ r = \text {target to detector distance} }[/math] [math]\displaystyle{ KE = \text {kinetic energy of alpha} }[/math] [math]\displaystyle{ θ = \text {scattering angle} }[/math]
Find the number of particles per unit area striking the detector given the following values: [math]\displaystyle{ N_i = 5 }[/math] alpha particles [math]\displaystyle{ n = 8.4866 * 10^{22} \text {atoms in 1} cm^3 }[/math] [math]\displaystyle{ L = 1 cm }[/math] [math]\displaystyle{ Z = 26 }[/math] [math]\displaystyle{ e = -1 }[/math] [math]\displaystyle{ k = 8.988 * 10^9 }[/math] [math]\displaystyle{ r = 10 cm }[/math] [math]\displaystyle{ KE = (1/2)*m*v^2 }[/math] where [math]\displaystyle{ v_a = 1.53 * 10^7 m/s \text {and mass of the alpha particle is} 6.64424*10^27 kg }[/math] [math]\displaystyle{ θ = 0.18 degrees }[/math]
Plug each number into the equation (make sure units cancel).
[math]\displaystyle{ N(0.18) = {\frac{5*8.4866*10^{22}*1*26^2*{(8.988*10^9)}^2*-1^4}{4*10^2*{((1/2)(6.64424*10^27){(1.53*10^-7)}^2)}^2*sin^4(0.18/2)}} }[/math]
= 1.57341 * 10^27 particles striking the surface per cm
Rutherford found that the fraction of particles scattered at an angle [math]\displaystyle{ θ }[/math] or greater can be modeled by the equation [math]\displaystyle{ F_{θ} ≈ e^{(−θ/θ^2_m)} }[/math] . At what angle would Rutherford have found a fraction of [math]\displaystyle{ 10^{45} }[/math] particles to be at that angle or greater than? ( [math]\displaystyle{ θ_m ≈ 1 }[/math] for a gold leaf foil)
Using the formula [math]\displaystyle{ F_{θ} ≈ e^{(−θ/θ^2_m)} }[/math] , we can rearrange to solve for θ by taking the log of both sides:
[math]\displaystyle{ log(F_{θ}) = −θ/θ^2_m }[/math]
Then, we can multiple by [math]\displaystyle{ -θ^2_m }[/math] to find:
[math]\displaystyle{ θ = −(θ^2_m)log(F_{θ}) }[/math]
Plugging in the given information, [math]\displaystyle{ θ = −(1^2)log(10^{45}) = 45 }[/math] . Therefore, a fraction of [math]\displaystyle{ 10^{45} }[/math] particles are scattered at about an angle of 45 degrees or greater.
A proton and an electron are a distance [math]\displaystyle{ {7.2*10^{-9}m} }[/math] apart. What is the electric potential energy of the system consisting of the proton and the electron?
[math]\displaystyle{ {U_{elec}} = {\frac{1}{4πε_0}}{\frac{q_{+}q_{-}}{r}} }[/math]
[math]\displaystyle{ {U_{elec}} = {9*10^{9}}{\frac {N*m^2}{C^2}}*{\frac{1.6*10^{-19}*(-1.6*10^{-19})}{7.2*10^{-9}}} = {-3.2*10^{-28}}{J} }[/math]
Connectedness
This topic is related to the study of chemical engineering. Without the discovery of the nucleus, any progress in this field would be limited based on the interaction of atomic particles. This would also hinder the medical field for very similar reason. Much of the understanding of sciences has its roots in the understanding of the atom and its functions. This experiment and the idea of atomic collisions helped to widen the atomic grasp. One of the best industrial examples of atomic collisions is the Large Hadron Collider.

Around the early 1900s, very little was known about atoms besides the ground breaking experiments conducted by J.J. Thompson in 1897. Thompson discovered what we call the electron. He hypothesized that electrons were negatively charged particles. It was also speculated that there must be a positive charge to balance out the negative charge from the electron. This "Plum Pudding Model" was invented by Thompson. This model assumed that matter consists of atoms which are overall positively charged, but with some type of negative electron charge throughout it. The electrons function as the "plum" which was evenly distributed through a positively charged "pudding".
With the knowledge of the plum pudding model of the atom, Ernst Rutherford and a small group of scientists set out to discover the properties behind alpha particles. The experiment, now known as the Gold Foil Experiment, was used to test this in 1911. It involved launching alpha particles at a small piece of gold foil. It was hypothesized that the alpha particle would be deflected at times, but at an angle because it was assumed that the alpha particle was more dense than the gold foil atom. They registered deflected particles through light emissions that would occur when the alpha particle hit the light source. Much to their surprise, some of the alpha particles they launched bounced straight back. This demonstrated that the gold particle was more massive than expected. It led to the discovery that the atom contained a positively charged nucleus. This was a major break through in the study of the atom in that it showed what the atom's composition was and how it act around other atoms.
Collisions is a related helpful page to get a foundation in collisions. Elastic Collisions and Inelastic Collisions are also useful, and Scattering: Collisions in 2D and 3D takes a broader look at the principles involved in the Rutherford Experiment.
Further Reading
Another page on Rutherford's Experiment.
External Links
MIT video of an experiment confirming Rutherford's model.
Chabay, R.W., & Sherwood, B.A. (2015). Collisions. In Fiorillo, J. Editor & Rentrop, A. Editor (Eds.), Matter and Interactions (383-410). John Wiley & Sons, Inc.
“Ernest Rutherford.” New Page 2, chemed.chem.purdue.edu/genchem/history/gold.html.
"History of Rutherford Experiment". HyperPhysics. Web. 03 Dec. 2015. Retrieved from: < http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html >.
“Rutherford Scattering.” MIT OpenCourseWare, MIT Department of Physics , ocw.mit.edu/courses/physics/8-13-14-experimental-physics-i-ii-junior-lab-fall-2016-spring-2017/experiments/rutherford-scattering/MIT8_13-14F16-S17exp15.pdf.
Navigation menu

IMAGES
COMMENTS
A replica of an apparatus used by Geiger and Marsden to measure alpha particle scattering in a 1913 experiment. The Rutherford scattering experiments were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. They deduced this after measuring how an alpha particle beam is scattered when ...
Rutherford gold-foil experiment In 1909 Rutherford disproved Sir J.J. Thomson's model of the atom as a uniformly distributed substance. Because only very few of the alpha particles in his beam were scattered by large angles after striking the gold foil while most passed completely through, Rutherford knew that the gold atom's mass must be ...
The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom. Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of ...
The Rutherford Gold Foil Experiment offered the first experimental evidence that led to the discovery of the nucleus of the atom as a small, dense, and positively charged atomic core. Also known as the Geiger-Marsden Experiments, the discovery actually involved a series of experiments performed by Hans Geiger and Ernest Marsden under Ernest ...
The Rutherford Scattering Experiment Tony Tyson, Maxwell Chertok, Chris Brainerd, Joseph Levine March 17, 2023 1 Introduction The foundations of modern ideas about atomic structure are considered to have been laid by Sir Ernest Rutherford in 1911, with his postulates concerning the scattering of alpha particles by atoms.
Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial University of Tokyo, first proposed the theory of the nucleus as it is known today. Rutherford's "gold foil experiment" led to the discovery that most of an atom's mass is located in a dense region ...
In the now well-known experiment, alpha particles were observed to scatter backwards from a gold foil. Rutherford's explanation, which he published in May 1911, was that the scattering was caused by a hard, dense core at the center of the atom-the nucleus. Ernest Rutherford was born in New Zealand, in 1871, one of 12 children.
Atom - Nuclear Model, Rutherford, Particles: Rutherford overturned Thomson's model in 1911 with his famous gold-foil experiment, in which he demonstrated that the atom has a tiny, massive nucleus. Five years earlier Rutherford had noticed that alpha particles beamed through a hole onto a photographic plate would make a sharp-edged picture, while alpha particles beamed through a sheet of mica ...
Rutherford's Gold Foil Experiment helped detect that there was a large positively charged mass in the center of an atom: the nucleus. ... but its existence wasn't confirmed until 1932 when James Chadwick proved it. A Mathematical Model. Rutherford modeled the effect the alpha particle has on the electrons of the gold atom. He did this by ...
After Rutherford's discovery, subsequent research determined the atomic structure which led to Rutherford's gold foil experiment. Scientists eventually discovered that atoms have a positively charged nucleus (with an atomic number of charges) in the center, with a radius of about 1.2 × 10 −15 meters × [atomic mass number] 1 ⁄ 3. Electrons ...