

Sticky Ice Science Experiment
Quick, easy and a little magical – this kid’s science experiment with ice is simply too cool not to do. The classic activity challenges little magicians scientists to lift an ice cube using only a string and salt . With serious wow factor, this science experiment is a simple, fun way to learn about salts’ effects on the freezing point of water.
Looking for more fun science kids will beg to repeat? Check out our 30 Science Experiments (complete with a no prep journal to record results) in our shop!

Getting Ready
Oh how I love simple activities – and this one delivers. I quickly grabbed a piece of string and three bowls. Next, I filled one bowl with a little water, one with ice and one with salt. Then, we were ready to make some ice experiment magic!

Ice Science Experiment
I called my 5 year-old over and asked her is she could lift a piece of ice from a bowl of water using only the string. I could tell she thought I must be up to something as she giggled and shrugged. I had her place a few ice cubes in the water and handed her the string. She placed it in the bowl and tried to lasso a cube without success.

Next I passed her the bowl of magic powder salt and asked her what she thought would happen if she sprinkled it on the ice. Having recently used salt to make homemade ice cream, A told me it would melt the ice. “Let’s see,” I replied. A sprinkled on the salt and it indeed melted the ice.

Next, I had A place the string on the ice and sprinkle salt over the string. Now came the hard part, waiting! We counted together to help pass the time.

“…58, 59, 60!” A carefully lifted the string with the utmost concentration and – voila!

I think her face says it all! She was so intrigued we repeated the magical ice science experiment 4 more times. While we waited during the other experiments, I explained to A what was happening we talked about other ways people use salt to melt ice (ice on roads, making ice cream), and whether the size of the salt would have an effect on the ice.
The Science Behind Sticky Ice
The salt sprinkled on the ice causes it to start melting, just as salt added to icy roads does in winter. Water freezes at 32 degrees F (0 degrees Celsius) but when salt touches the ice, it lowers the freezing point to much lower than that. In order for the ice to melt, however, it has to absorb heat. The heat that it absorbs comes from what’s around it, which in this case was the water near the ice cube. Some of this water becomes so cold that it refreezes and the string becomes trapped. The string is now stuck and you can lift the cube out of the water.
- Try using different sized salt {rock salt, epsom, and table salt} to see if has an effect on the time it take the water to refreeze around the string.
- Try using a different type of salt {sodium bicarbonate aka baking soda} to see if it is as effective as table salt {sodium chloride}
- Set up a control bowl of just ice and water as well as the salty ice water, use a thermometer or simply your finger to see if the addition of salt lowers the overall water temperature.
- Use salt and ice to make homemade ice cream .
Be sure you check out our awesome 30 Science Experiments just for kids!
69 Comments
- Pingback: 40 Finest Winter Science Experiments for Youngsters of All Ages | Smart Online Life
- Pingback: ≫ 70 experimentos científicos fáciles con materiales que ya tienes
- Pingback: 40+ Easy Science Experiments For Students: Lots Of Great Ideas
- Pingback: 35+ Easy Science Experiments for Kids to Try at Home – Giftable
- Pingback: 35+ Easy Science Experiments for Kids to Try at Home - Giftable
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- free-science-experiments
- ice-cold-magic
Ice Cold Magic

When the sun is shining it feels great to pop some ice in your drink, right?
Well, next time you cool a beverage take 30 seconds and try out this simple but magical experiment.

What Do I Need?
- Glass of water
- Plate (optional but makes it less messy)

How Do I Do It?
STEP1 - Challenge a friend or family member and see if they can lift up an ice cube just by placing a piece of string on it?
STEP2 - Wet your string and push it onto your ice cube. Can you lift it up?
STEP3 - No matter what they try it isn’t possible to lift your ice cube up with just a piece of string. That’s where the science comes in!
STEP4 - Make sure your string is wet and place it on top of your ice cube. Then sprinkle half a tea-spoon of salt on top of your string.
STEP5 - Wait for 30 seconds...
STEP6 - Slowly and gently lift your string into the air and watch (in amazement!) as the ice cube lifts up with it.
STEP7 - Have a good think about what’s going on!?!

What’s Going On?
That certainly looked magical but there’s some real science going on. It was only possible to lift up our ice cube when we added our salt. So what is it about adding the salt that made the difference?
The salt lowers the freezing point of the water and this means that the ice around the string melts. That’s why in the winter we put salt on the roads, as it causes the ice to melt!
When the ice freezes over again it freezes around the string and connects our string to our ice cube. As long as we’re really careful when we lift our string we should be able to “magically” lift our ice cube too!

More Fun Please! - Experiment Like A Real Scientist!
- What type of salt works best?
- Does it make any difference?
- What could you use instead of string?
- What’s the biggest ice cube that you can lift?

Want A FREE Science Experiment Book?
If you like fun science experiments then you'll love this FREE Science experiment book....
It's packed full of awesome experiments you can do at home with "stuff" you've already got.
Your FREE Science experiment book is:
- 5 Star Reviewed
- Dragons' Den Approved
- Yours completely FREE.
Click Here to download your FREE copy now!

Kids Party Coming Up?
If you like fun science experiments then you’ll love the Dragons’ Den winning Sublime Science Party. Check price & availability in your area and grab your FREE Kids Party Survival Guide right now!

Home - About - Privacy Policy - Terms & Conditions - Facebook - Instagram - LinkedIn - Twitter - Sitemap - Blog - Contact
Copyright 2008-2023 - Sublime Science - All Rights Reserved - Registered in England and Wales, Company Number 6680269. Registered Address: The Sublime Science Lab (Unit4) Fernleigh Business Park, Blaby Rd, Enderby, Leicester LE19 4AQ - Call: 0116 380 0750
You Just Couldn't Resist!
There's some serious science behind why you just clicked!
If you'd like more awesome science experiments then enter your email address below and grab your FREE science experiment book!
You'll get your FREE copy of 'Don't Eat Your Slime', weekly 'Top Secret Science Experiment Printables' and occasional awesome offers. Happy experimenting!
(enter your email address below to get your FREE science experiment book)
YES, I Want My FREE Experiment Book!
( You'll get your FREE copy of 'Don't Eat Your Slime', weekly 'Top Secret Science Experiment Printables' and occasional awesome offers. Happy experimenting!

- Uncategorized
- Space science
- States of matter
- Mixtures and solutions
- Light and optics
- Forces and motion
- Electricity
- The animal kingdom
- Habitates and ecosystems
- valentine's day
- St. Patrics day
- Back to school
- 4th of July
- Experiments
- Weather and climate
- Earth's Structure
- Crafts and DIY
- Class managements
Get the Latest Straight to Your Inbox:

This is an amazing science trick that allows you to melt part of an ice cube and use it to pick up and move the whole frozen block. It’s a simple experiment that demonstrates the power of chemistry and phase changes. With just ice, salt, and a piece of string, you can “stick” a string to an ice cube and lift it up!
The experiment visually reveals the effect salt has on ice – allowing you to essentially glue a string to an ice cube!
So get ready to astound your students as you appear to defy physics and pick up an ice cube with a piece of string. This is an unforgettable experiment that really hammers home some core scientific principles in a hands-on way!
Materials :
- Cup of water
What to do?
- Place an ice cube in a cup of water.
- Lay the yarn over the ice cube.
- Sprinkle salt on top of the yarn.
- Wait one minute.
- Lift up the yarn.

How does this happen?
Salt causes ice to melt – or more precisely, makes it harder for water to freeze. In an ice cube, water molecules are constantly transitioning between liquid and solid states. We don’t see this because only a small amount of water makes this transition at a time, so the ice cube appears unchanged. Adding salt causes water molecules that have turned to liquid to have difficulty freezing again, effectively melting the ice. In this experiment, we add a small amount of salt over the yarn, which melts the ice at that point. Over time, the salt disperses and its concentration decreases, allowing the water above the yarn to freeze again. This re-freezes the water-soaked yarn, sticking it to the ice cube.
Looking for more Winter science? <<More Winter Science Here>>
We’d love to showcase your creativity, share pictures of your experiments with us, and together, we can inspire young scientists everywhere.

View my latest posts:

Back to School Checklist for Science Teachers: Setting Up for Success

Engaging Science Learning through Games

Water cycle booklet

Albedo in Action: Balloon Pop Test
Like & follow:, stay in touch: [email protected].

Lets stay in touch!

“Subscribe now and never miss out on our exciting science experiments and educational insights!”
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School

The Best Winter Science Experiments
January 2, 2024 By Emma Vanstone Leave a Comment
Winter is a great time of year to try some science at home . Ice and snow-themed experiments don’t require special materials, look great, and if you can do them outside, are almost mess free!
I’ve pulled together a collection of what we think are the best winter science experiments for kids, including a free checklist you can download at the bottom of the post.
My FREE winter science eBook is a great place to start with easy-to-follow instructions for five winter science investigations .

The Best Winter Science Experiments for Kids
Snow volcano.
First up is a snow volcano . These are super easy to make. Gather snow around a bottle and add water, food colouring, dish soap ( washing up liquid ) and baking soda. Try making different colours of lava using food colouring and experiment to find the best fizzy recipe!

Ice and salt investigation
Melting ice is always fun and super easy to set up.
Younger children usually enjoy ice excavations , and ice and salt experiments are great for older kids.
Ice investigations work well in summer as an outdoor science activity and in winter when it’s icy outdoors, too!

How to make a frozen bubble
It needs to be extremely cold to make a frozen bubble, but it’s worth a try if you get a cold snap. Fireflies and Mudpies show you how it’s done.

Frost on a can
This ice and salt investigation using a tin can is wonderfully visual. It’s even more fun if you turn the can into a frosty snowman ! Frost quickly appears outside the can, making it an excellent classroom science demonstration.

How do polar animals stay warm
Find out how polar bears and penguins stay warm in extreme temperatures using lard! Take care with this activity as the icy water can get very cold.

Another activity linked to polar animals is this ‘ Why don’t penguins freeze ?’ activity, which uses wax crayons to show how the waxy feathers of a penguin help them stay dry.

Snowman Catapult
Lolly stick catapults are easy to make and lead to many investigative opportunities. Make different-sized catapults and investigate to see how far different objects fly. We made a snowman themed catapult , but you can change the theme for any topic or time of year.

Ice Decorations
Ice decorations are lovely to hang off a tree in winter. Fill them with nature items or even small plastic toys.
Children can then observe how the decoration changes during the day or when the outside temperature increases.

Pretend Snow
Fake snow is excellent for all kinds of sensory play and activities. I made three different types, so have a go and choose your favourite.

Lift ice with a string
Freeze some water, put the ice into a bowl and pour a little water over the top. Place the string on the surface of the ice and sprinkle it with salt. Leave for a few minutes, and then gently try to lift the string. Find out how many ice cubes you can lift with one string .

Winter print and play science pack
My winter themed print and play science experiment pack contains five simple paper-based science activities, including jumping snowflakes, penguin shadow puppets and a water drop maze.
I also have lots of winter STEM Challenges you might like to try.
The best winter science experiment checklist!
Download my winter science experiment checklist and try them all!

Last Updated on January 19, 2024 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
January 25, 2018
Lift Ice Cubes with Chemistry
An Ice-Cold Activity from Science Buddies
By Science Buddies & Sabine De Brabandere
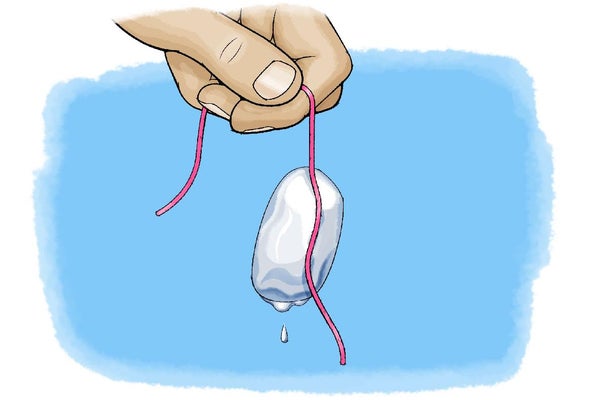
Melt and freeze your way to some science magic--all it takes is a dash of salt!
George Retseck
Key concepts Chemistry Freezing Melting Freezing-point depression
Introduction Did you ever wonder why they use salt to de-ice roads? Did you know that snow more readily sticks to pavement treated with salt? Why would this be the case? In this activity you will use the same principles to pick up ice cubes with a string. Is it possible to do this—without getting your hands cold? Do the activity and see what a pinch of salt can do!
Background Water is made up of tiny building blocks called molecules. These water molecules don’t sit still—they wiggle and move around all the time. The water’s temperature is a measure of how much these molecules move. When you cool water you lower its temperature, and the molecules slow down. Eventually after you cool the water enough they move so little they can form strong connections and the water freezes, turning into ice, which is a solid. For pure water this transition happens around 0 degrees Celsius (32 degrees Fahrenheit). Conversely, if you add heat to a block of ice, its molecules will start wiggling more; eventually they move around too much to stay stuck together, and the ice melts, turning back into a liquid. These transitions, however, do not happen instantaneously—water and ice can coexist. (That is, a whole ice cube does not turn to liquid water all at once.) To visualize this process you can think of a group of excited children—like molecules at higher temperature, they move and wiggle a lot. The higher their excitement, the more they move. Calm them and they will slow down just like molecules slow down when you reduce the temperature. Eventually you might get these children to hold hands and stand nicely in line—so they behave like a solid.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
When the water is not pure, the water molecules cannot connect as readily to form a solid; other particles get in the way. That is why salty, sugary or other water solutions’ freezing points—temperatures at which they turn solid—are lower than 0 degrees C. This explains why treating roads with salt in winter can prevent them from icing over. The salt dissolves in the water, lowering its freezing point, which will only turn to ice at temperatures well below 0 degrees C. In this activity you will use this characteristic in a clever way to pick up an ice cube.
Three glasses
Three strings, each about 20 centimeters long (Yarn works well.)
One sticky note
Watch or timer
Thermometer (optional)
Preparation
Gather all of your materials on a work surface that can tolerate spills.
Fill your three glasses with cold water
Put a few ice cubes in the first glass of water. They float because ice is less dense than water.
Search for a cube whose surface is at about water level. Lay one end of a piece of string across that ice cube. Do you think the ice will stick to the string?
Lift the string. Does the ice cube stick to the string? Why do you think this is the case?
Do you have ideas on how you can lift the ice cube with a string, without touching the cube with your hands?
Try again, but now, sprinkle some salt over the string and ice cube. Do you think the ice will stick to the string if you lift it?
Lift the string. Does the ice cube stick to the string? After all, salt is not glue, right?
What do you think would happen if you left the ice cubes with the strings on top out for a few minutes?
Take the two other glasses filled with water, add a few fresh ice cubes to each glass.
For both glasses, lay one end of a piece of string across an ice cube whose surface is about water level; let the other end of the string hang over the edge off the glass.
Sprinkle salt over the string and ice cube in one of the glasses, and mark this glass with a sticky note. Wait for about two to three minutes. Do you think the ice will stick to the string after you give it some time? Would both, only one or none stick? Why would this happen?
After about two to three minutes, lift one string at a time. Can it pick up the ice cube? Can you explain what you see? If you cannot pick up any ice cube, try again but wait a little longer this time.
Extra: Try some other substances, such as sugar or food coloring. Can these make the ice cube stick to the string?
Extra: Make two identical water-and-ice baths. Add more salt to one bath, leave the other as is. Measuring the temperature of each water-and-ice bath every minute for the next five minutes. Graph your results. What do you observe? Is one colder than the other? Why would that be?
Extra: Can you re-create a mini iceberg in a saltwater ocean in a bowl at home? What temperatures can prevent your “ iceberg ” from melting and “ ocean ” from freezing?
Observations and results Could you lift the ice cube you had sprinkled with salt and left untouched for few minutes? Did you fail to pick up the cube in all the other cases? Why does this happen? First, the ice around the string melts when you sprinkle it with salt, then the string freezes to the ice cube.
You probably wonder why it happens only when you sprinkle salt over the ice cube and string. When you sprinkle salt over ice it dissolves in the thin layer of water above the ice. Because saltwater freezes at a lower temperature than pure water adding the salt makes some ice melt and absorb heat in the process. The area just around it thereby cools and freezes water molecules to the ice cube, also freezing the string on. Without the salt, the water and ice are both at the same temperature and the string does not freeze to the ice. In both cases the ice cube gradually melts as it absorbs heat from the air around it, but without the salt the string cannot freeze to the cube.
If you used sugar, you would see the same effect. The cube sticks to the string. Dissolving other substances in water will also lower the freezing point and create the same effect.
Cleanup Pour the water and ice cubes in the sink, and wash the glasses.
More to explore Scrumptious Science: Making Ice Cream in a Bag , from Scientific American High Seas: What Happens When the Glaciers Melt? , from Scientific American Freezing-Point Depression , from CK–12 Freeze Your Fruit with Science , from Scientific American Science Activities for All Ages! , from Science Buddies
This activity brought to you in partnership with Science Buddies


- Foundations
- Write Paper
Search form
- Experiments
- Anthropology
- Self-Esteem
- Social Anxiety

- Kids' Science Projects >
Lifting Ice Cube Experiment
The Lifting Ice Cube Experiment is a trick that will let you lift ice cubes without getting your hands wet or making use of a spoon! You don't believe it is possible? With science, nothing is impossible!
This article is a part of the guide:
- Kids' Science Projects
- Paper Towel
- Salt Water Egg
- Fruit Battery
Browse Full Outline
- 1 Kids' Science Projects
- 2 How to Conduct Science Experiments
- 3.1 Mold Bread
- 3.2 Popcorn
- 3.3 Salt Water Egg
- 3.4 Corrosiveness of Soda
- 3.5 Egg in a Bottle
- 3.6 Fruit Battery
- 4.1 Pendulum
- 4.2 Paper Towel
- 4.3 Paper Airplane
- 4.4 Charge a Light Bulb
- 4.5 Lifting Ice Cube
- 4.6 Magic Egg
- 4.7 Magic Jumping Coin
- 4.8 Invisible Ink
- 4.9 Making-a-Rainbow
- 4.10 Oil Spill
- 4.11 Balloon Rocket Car
- 4.12 Build an Electromagnet
- 4.13 Create a Heat Detector
- 4.14 Creating a Volcano
- 4.15 Home-Made Glue
- 4.16 Home-Made Stethoscope
- 4.17 Magic Balloon
- 4.18 Make a Matchbox Guitar
- 4.19 Make Your Own Slime
- 5.1 Heron’s Aeolipile
- 5.2 Make an Archimedes Screw
- 5.3 Build an Astrolabe
- 5.4 Archimedes Displacement
- 5.5 Make Heron’s Fountain
- 5.6 Create a Sundial
The Lifting Ice Cube experiment demonstrates the melting and freezing properties of water, which results to another property of matter called adherence. Adherence refers to close physical attachment or union of two objects. To further understand this phenomenon, let us do an experiment!

In this experiment, you will need the following materials:
- Glass of water

Drop an ice cube in the glass of water. Take the string and dangle the end of it on the ice cube, and then keep it still.
While the string is dangled down onto the ice cube, sprinkle a bit of salt on the ice cube. Set aside for a few minutes. After some time, try to lift the string and observe what happens to the ice cube.
In the Lifting Ice Cube experiment, notice that adding salt onto the ice cube caused it to attach itself to the string, allowing you to lift it out of the glass just with the use of the string; like fishing! Read on to find out how this happened.
Before we explain how this happened, let us talk about the freezing point of water first, and the melting point of ice. The freezing point of water and the melting point of ice under normal conditions is 0°C or 32°F.
How it Happens
When you placed the ice cube in the glass of water, two processes started to occur: the ice started melting into the water and the water started to freeze. Because the two processes have been happening simultaneously, we can say that the ice and the water are in dynamic equilibrium. Here, the rate of freezing and the rate of melting is the same. As ice melts, the ice molecules begin to escape into the water. On the other hand, when the water freezes, its molecules are captured on the ice surface. With this happening at the same time, it is safe to say that there are no changes created in either the ice or the water. This state of equilibrium shall stay as long as the water keeps its temperature of 0°C (32°F).
When we sprinkle salt on to the ice cube, the state of equilibrium is disrupted. The salt molecules dissolve and join the water molecules thus changing the water's rate of freezing. The rate of melting is now much faster than freezing hence causing the ice to melt. However, to be able to restore equilibrium, the water's freezing point drops causing the ice to freeze out of the salt water. The salt starts to crystallize and ice refreezes around the string. This causes the ice cube to stick to the ends of the thread enabling you to pick it up just by lifting the string!
- Psychology 101
- Flags and Countries
- Capitals and Countries
Explorable.com (Nov 3, 2011). Lifting Ice Cube Experiment. Retrieved Nov 26, 2024 from Explorable.com: https://explorable.com/lifting-ice-cube-experiment
You Are Allowed To Copy The Text
The text in this article is licensed under the Creative Commons-License Attribution 4.0 International (CC BY 4.0) .
This means you're free to copy, share and adapt any parts (or all) of the text in the article, as long as you give appropriate credit and provide a link/reference to this page.
That is it. You don't need our permission to copy the article; just include a link/reference back to this page. You can use it freely (with some kind of link), and we're also okay with people reprinting in publications like books, blogs, newsletters, course-material, papers, wikipedia and presentations (with clear attribution).
Want to stay up to date? Follow us!
Get all these articles in 1 guide.
Want the full version to study at home, take to school or just scribble on?
Whether you are an academic novice, or you simply want to brush up your skills, this book will take your academic writing skills to the next level.

Download electronic versions: - Epub for mobiles and tablets - For Kindle here - PDF version here
Save this course for later
Don't have time for it all now? No problem, save it as a course and come back to it later.
Footer bottom
- Privacy Policy

- Subscribe to our RSS Feed
- Like us on Facebook
- Follow us on Twitter

IMAGES
VIDEO
COMMENTS
The classic activity challenges little magicians scientists to lift an ice cube using only a string and salt. With serious wow factor, this science experiment is a simple, fun way to learn about salts’ effects on the freezing point of water.
In this ice and salt demonstration, an ice cube is lifted from a glass of water attached to a string! Salt is used to melt the ice, which then refreezes around the string. Glass or jar. Water. Ice cube tray. Food colouring – optional. Salt. String or wool/yarn. Freezer.
It explains why treating roads with salt in the winter can prevent them from icing over. The salt dissolves in the water, lowering its freezing point, which will only turn to ice at temperatures well below 0 degrees C. In this activity, you use this characteristic in a clever way to lift an ice cube.
When the sun is shining it feels great to pop some ice in your drink, right? Well, next time you cool a beverage take 30 seconds and try out this simple but magical experiment. What Do I Need? How Do I Do It? string on it? STEP2 - Wet your string and push it onto your ice cube. Can you lift it up?
It’s a simple experiment that demonstrates the power of chemistry and phase changes. With just ice, salt, and a piece of string, you can “stick” a string to an ice cube and lift it up! The experiment visually reveals the effect salt has on ice – allowing you to essentially glue a string to an ice cube!
My son lifted an ice cube from a cup of water with just a string touching it. He was elated and in awe as the cube dangled in the air. All you need is a cup of water, an ice cube, a string and salt.
Find out how many ice cubes you can lift with one string. Winter print and play science pack My winter themed print and play science experiment pack contains five simple paper-based science activities, including jumping snowflakes, penguin shadow puppets and a water drop maze.
In this activity you will use this characteristic in a clever way to pick up an ice cube. Materials. Three strings, each about 20 centimeters long (Yarn works well.) Preparation. Gather all of...
Lift an Ice Cube with String! - Science Experiment - Melting Point and Freezing Point of Water. See More at http://www.MathTutorDVD.com.Learn how to lift an ice cube from a glass of water...
The Lifting Ice Cube Experiment is a trick that will let you lift ice cubes without getting your hands wet or making use of a spoon! You don't believe it is possible? With science, nothing is impossible!