

Eggshell in Vinegar Experiment
Eggshells are quite strong, even though they are considered fragile. Birds have evolved to make eggs that are hard to break. Of course, that doesn’t mean they can survive being dropped from a height, or being smashed with a hammer. But, the calcium carbonate that makes up an eggshell is very tough.
A healthy bird’s body produces calcium carbonate that coats the soft membrane and yolk just before the egg is ready to be laid. But what happens if the chemical prevents the thick coating of calcium carbonate around the egg from forming?
We can do an experiment, using ordinary kitchen vinegar (dilute acetic acid), and see what happens to the calcium in an eggshell when it is exposed to an acid.
A clear glass A raw egg A bottle of kitchen vinegar A spoon
- Fill the glass with vinegar. Leave enough empty space that you can put the egg in without displacing any vinegar.
- Observe the outside of the egg. Carefully feel it– what does the shell feel like? Can you gently scratch it with your fingernail? Does anything happen?
- Write a prediction in your science journal. What do you think will happen to the egg in the vinegar? Will it change or not? How might it change? How long do you think it will take before you notice a change?
- Carefully put the egg into the glass of vinegar. You may need to use a spoon to help. The egg may float in the liquid for a minute or two, but it will eventually sink. Record the time you put the egg in the vinegar, then observe the egg for a few minutes. Do you notice anything different yet?
- Watch for bubbles on the surface of the eggshell. What do you think might be causing the bubbles? Write down your prediction in your science journal.
- Let your egg stay in the vinegar for at least an hour, before checking again. Record the time and any new changes to the egg. Are there more bubbles? Are there any other changes?
- Every few hours, you can check the egg. Be sure to record the time and any new changes in your science journal, along with any new predictions or questions you have.
- Eventually, you will see a frothy layer of white foam collecting on top of the vinegar. This is made from layers of the calcium carbonate from the eggshell. In about 24 hours, draw a picture of what the egg looks like now. How has it changed? What do you think will happen if you leave it in the vinegar?
- You can carefully remove the egg and rinse it, and gently scrape the eggshell with your fingernail again. What happens? Be careful, the eggshell will be a lot weaker!
- If you leave the egg in the vinegar for about 36 hours, eventually all the calcium carbonate will be dissolved by the acetic acid, leaving just the soft membrane and yolk behind. Do you think an egg would be able to hatch if its eggshell was a lot weaker and softer? Why not?
Think Like A Scientist: Explore More
You can expand on this investigation by taking your decalcified egg and seeing what happens when you put it into another liquid.
Carefully remove the decalcified egg from the vinegar, and very gently rinse it off with plain water. Be careful– it will be fragile, and might break!
Fill a glass with a different liquid. You could try corn syrup, salt water, soda, or plain water, or something else. Make sure that an adult says it’s all right to experiment with chemicals and kitchen items.
Place the decalcified egg in the new liquid, and record the time you put it in. Now, predict what will happen to the egg in the new liquid, and write it in your science journal. Leave the egg in the new liquid for at least 1 hour before you check on it. Has anything changed? Record the changes and the time in your science notebook.
Check on the egg again in 12 hours. Record the time, any changes, and any new questions you have in your science notebook.
Do you think that there is any substance that can regrow the calcium eggshell once it’s been weakened? Why or why not?
Some pesticides, like DDT, build up in birds’ bodies when they eat other animals that have eaten it. The chemical doesn’t pass through the bird’s body, but stays, and changes how the bird’s body functions– one change makes it harder for them to produce as much calcium. How do you think this might affect birds and their babies?

Hands-On Education Experiences
Founded in 2002 as a private response to a crisis in public science education, ScienceWorks is committed to inspiring wonder and stimulating creative exploration through fun interactive science.
Upcoming Events Calendar
ScienceWorks offers a variety of programs, for curious minds of all ages, that are both fun and educational. Check out our calendar and plan a visit today!
Sign Up to Stay In the Know

Naked Egg (Dissolving Egg Shell) Experiment
- April 30, 2019
- 3-4 Year Olds , 5-6 Year Olds , Chemistry , Household Items , Popular
Simple science experiments for kids: Do you want show naked egg to your kids? Try this fun project by dissolving an eggshell into a glass of vinegar solution.

Try this simple activity which involves absolutely no complication but only fun. And you know what? There is no need to run around to shop for materials because you can find everything from your household items . Trust me, we tried this experiment couple of time in the last 15 days as kids kept on insisting to watch the reactions. Also, my little princess Tisha did not allow the result show and interrupted every time out of curiosity.
Dissolving the Eggshell from an Egg & Making Transparent Egg
What is required?
- Vinegar (White )

- Place the egg in the glass jar
Make sure your jar is not heavy and let kids handle them easily. Ask them to place the egg into the glass jar. Ensure it does not break and provide help to kids. Preferably use a wide-mouthed glass so that easily hands can go inside. Also, there will be rooms for the egg to swell.
- Mix Vinegar Solution Now, take vinegar and transfer that to the jar until it covers the entire egg. Finally, add few more drops to make sure that eggs are floating. Initially, the egg will float when it is fresh and then sink.

The fun begins quickly after adding the vinegar to the jar and you can witness minute bubble formation on outer layer of the egg. This is due to the release of CO 2 (carbon dioxide). Imagine the fizzy sound when opening any aerated juice. This is similar to that chemical reaction which happens n the jar.

- Close the jar and keep it aside for a maximum of 7 days
Osmosis takes places making the egg to swell and also the color of the eggshell fades from brown. A scummy layer is formed on the surface and it is good to change the Vinegar solution when you witness this layer formation. However, it is optional and does not bother if you forgot to do it or just don’t have time.
- It’s time to empty the vinegar from the jar and take the egg outside. Wash off the residual eggshell.
Wow! You can see the naked egg without eggshell. It is now soft like a sponge and light in weight. In case, the eggshells are hard to remove, then don’t panic it needs more days and some more vinegar. So wait with patience to enjoy watching and touching the naked egg soft as a sponge. 🙂
These are cool to look at and also you can find the intact membrane inside. It feels rubbery to touch and squeezing them gently adds fun. It is now possible to see through the lucid egg which contains the yolk. Also, they are seen floating on the top.

The reaction between an acid solution and a base can be demonstrated using this activity. The acetic acid present in vinegar reacts with the shell which is nothing but calcium carbonate. Therefore vinegar is acidic and eggshell is the base.
The reaction between acetic acid + calcium carbonate forms a compound that is soluble in water and named as calcium acetate. Along with this CO 2 is released. Thus the eggshell gets dissolved and the inner membrane remains unaltered giving an appearance of an exposed egg.
Tisha could not understand the science but activity took part in the experiment. She is too small for the explanation part. She expressed interest to carefully take an egg from the refrigerator and drop in the glass jar. Then she added vinegar to the egg in the jar and closed the jar with the lid. She enjoyed watching the bubbles. She counted days and waited to take the egg out of the jar and wash to remove the residual shells. She was amazed at the texture of the egg and ready for a camera shoot. I am glad she is so involved but at the cost of breaking some eggs. Still, she enjoyed.
Therefore, we discussed the texture of the egg from the beginning of the experiment (hard) until the end (spongy). All the time during the discussion Tisha was busy meddling with the egg squeezing softly and bouncing on the floor.
However, Pritika being elder started asking questions of how and that led to learning a bit of chemistry and chemical reactions. Though she is not old enough to understand reactions but certainly gained knowledge about the science involved in this experiment. She got an idea about elements, molecule, etc. She now knows that elements make up the molecules and the entire world has elements in it.
We then discussed molecules, acids, base, and reactions. Also, she understood that lemon contains citric acid. Out stomach has some acids to digest food etc. Also, she understood that acid reacting with the base like baking soda will make a fizzy sound. She knows the uses of baking soda because of our “making of Anzac Biscuits ” experiment. We also did the erupting volcano experiment a month ago which helped her to understand this one easier.
But today she saw vinegar reacting with a base other than baking soda and understood the chemical reaction. During the entire process, I explained to her about vinegar, its properties, and eggshell properties, etc. Also, told her how CO 2 bubbles escaped, etc. This is my small initiative to expand her knowledge with practical experiments.

Do not view the activity as edible. As we have used vinegar to dissolve eggshell formation bacteria will not be a cause for concern. Egg absorbs the vinegar and this process is osmosis. However, we advise you to not use this egg in cooking.
We have extended this experiment to make colorful Rainbow Rubber Eggs

If you are interested in more dissolving egg experiments, refer to the below links:
- Making Naked Eggs | Explanatorium
- Bouncy Eggs | Childhood101
- How to Make a Naked Egg (video) | Imagination Station Toledo
- 20+ Science Experiments with Eggs
Or if you are just looking for a science project for upcoming easter, here are some:
Create your own Easter Egg shaped bubble wands (identify what shape bubbles will come out )
Do follow our Go Science Girls board on pinterest to know upcoming science experiments.
Subscribe to our newsletter , we will send you our latest experiments right to your inbox. We would love to have you on board with us!

- Plant Biology
- Human Biology
- Biology Cells Osmosis Experiment
Osmosis Experiment: Dissolving Egg Shells With Vinegar
How does osmosis keep you healthy.
Right now, as you read this, there are millions of things happening throughout your body. The food you ate just a bit ago is making its way through a watery slurry inside your stomach and small intestines. Your kidneys are working hard to excrete waste and extra water. The lacrimal glands near your eyes are secreting tears, which allow your eyelids to close without damaging your eyeballs. What’s one thing that all of these processes have in common? They all rely on osmosis: the diffusion of water from one place to another.
Osmosis factors heavily in each of these processes and is an important force for keeping every single cell in your body healthy. Osmosis is hard to see without a microscope. But if we create our very own model of a cell, using a shell-less chicken egg, we can see what happens when we manipulate the osmotic balance in the “cell”!

- 3 glasses (large enough to fit the egg plus liquid)
- 3 butter knives
- White vinegar (about 3 cups)
- Distilled water (about 2 cups)
- Light corn syrup (about 1 ¼ cups)
- Slotted spoon
- Measuring cup (1 cup)
- Measuring spoons (1 tablespoon and ½ tablespoon)
- Sticky notes and marker
- Scale (optional)
Note : It’s okay to touch the eggs, but remember to wash your hands afterwards to avoid any nasty surprises!
1. Place one egg in each glass. Pour in enough vinegar to cover each egg. Bubbles will start to form around the egg, and it’ll float up. To keep it submerged, put a butter knife in the glass to hold it down.
2. Put the three glasses in the refrigerator and allow to sit for 24 hours.
3. Gently holding the egg in the glass, pour out the old vinegar. Replace with fresh vinegar, and let sit in the refrigerator for another 24 hours. Repeat this process until the shells are fully dissolved and only the membrane remains. This should take about 2-3 days.
4. Gently remove the eggs using the slotted spoon and rinse with tap water in the sink. Rinse out the empty glasses as well.
5. Gently put the shell-less eggs aside for a moment on a plate.
6. Prepare three different sugar-water solutions as follows, labeling with sticky notes:
Glass 1: Label “hypertonic”. Pour in one cup of corn syrup.
Glass 2: Label “isotonic”. Add 1 ½ tablespoons corn syrup to the one cup measuring cup, and fill the remainder with distilled water. Pour into glass (make sure you get all the corn syrup out!) and stir to dissolve.
Glass 3: Label “hypotonic”. Pour in one cup of distilled water. Gently put one shell-less egg in each of the glasses, and let sit in the refrigerator for another 24 hours.
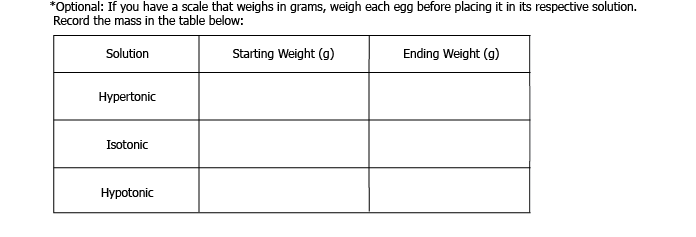
7. Remove the glasses from the refrigerator, and gently put the eggs on a plate. If you weighed the eggs before putting them in each solution, weigh them again. What happened to each of the eggs?

How does osmosis work?
Osmosis is the scientific term that describes how water flows to different places depending on certain conditions. In this case, water moves around to different areas based on a concentration gradient , i.e. solutions which have different concentrations of dissolved particles ( solutes ) in them. Water always flows to the area with the most dissolved solutes, so that in the end both solutions have an equal concentration of solutes. Think about if you added a drop of food dye to a cup of water – even if you didn’t stir it, it would eventually dissolve on its own into the water.
In biological systems, the different solutions are usually separated by a semipermeable membrane , like cell membranes or kidney tubules . These act sort of like a net that keeps solutes trapped, but they still allow water to pass through freely. In this way, cells can keep all of their “guts” contained but still exchange water.
Now, think about the inside of an egg. There’s a lot of water inside of the egg, but a lot of other things (i.e. solutes) too, like protein and fat. When you placed the egg in the three solutions, how do you think the concentration of solutes differed between the inside of the egg and outside of the egg? The egg membrane acts as a semipermeable membrane and keeps all of the dissolved solutes separated but allows the water to pass through.

How did osmosis make the eggs change size (or not)?
If the steps above work out properly, the results should be as follows.
In the case of the hypertonic solution, there were more solutes in the corn syrup than there were in the egg. So, water flowed out of the egg and into the corn syrup, and as a result the egg shriveled up.
In the case of the isotonic solution, there was roughly an equal amount of solutes in the corn syrup/water solution than there was in the egg, so there was no net movement in or out of the egg. It stayed the same size.
In the case of the hypotonic solution, there were more solutes in the egg than in the pure water. So, water flowed into the egg, and as a result, it grew in size.

Osmosis and You
Every cell in your body needs the right amount of water inside of it to keep its shape, produce energy, get rid of wastes, and other functions that keep you healthy.
This is why medicines that are injected into patients need to be carefully designed so that the solution has the same concentration of solutes as their cells (i.e. isotonic). If you were sick and became dehydrated, for example, you would get a 0.90% saline IV drip. If it were too far off from this mark it wouldn’t be isotonic anymore, and your blood cells might shrivel up or even explode , depending on the concentration of dissolved solutes in the water.
Osmosis works just the same way in your cells as it does in our egg “cell” model. Thankfully, though, the semipermeable membrane of the egg is much stronger, so you don’t have to worry about the egg exploding as well!
Related Topics
Choose one of the following categories to see related pages:.
- Experiments
Share this Page
Lindsay graduated with a master’s degree in wildlife biology and conservation from the University of Alaska Fairbanks. She also spent her time in Alaska racing sled dogs, and studying caribou and how well they are able to digest nutrients from their foods. Now, she enjoys sampling fine craft beers in Fort Collins, Colorado, knitting, and helping to inspire people to learn more about wildlife, nature, and science in general.
- Basic Types of Cells
- Cell Organelles
- Connective Tissue Cells
- Epithelial Cells
- Introduction to Cells
- Muscle Cells
- Nerve Cells
- Osmosis Experiment
- Structure of the Cell Nucleus
- Structures of the Cell Cytoplasm
Science Newsletter:
Full list of our videos.

Teaching Biology?

How to Make Science Films

Read our Wildlife Guide

New From Untamed Science


IMAGES
VIDEO